Avicenna J Dent Res. 14(3):137-143.
doi: 10.34172/ajdr.2022.25
Review Article
Diagnostic Strategies for Early Detection of Oral Squamous Cell Carcinoma: A Review Article
Soussan Irani 1, 2, * 
Author information:
1Associate Professor, Dental Research Centre, Oral Pathology Department, Dental Faculty, Hamadan University of Medical Sciences, Hamadan, Iran
2Lecturer at Griffith University, Gold Coast, Australia
Abstract
Background: Oral squamous cell carcinoma (OSCC) is the sixth most common cancer with high morbidity and mortality rates. Late diagnosis and high incidence rate of OSCC have become global healthcare issues. The purpose of this review was to provide an overview of the diagnostic and prognostic biomarkers in OSCC.
Methods: A literature search in PubMed, ScienceDirect, and Google Scholar was performed. The keywords "early detection", "oral cancer", and "oral squamous cell carcinoma" were searched in the title and abstract of the articles published in English from 2000 to mid-2021. The full texts of 250 articles were retrieved and only 63 articles met the inclusion criteria.
Results: In summary, all selected papers discussed the importance of early detection along with different factors and techniques to detect oral cancer. The biomarkers were divided into three groups as follows: salivary biomarkers, circulating biomarkers, and tissue biomarkers.
Conclusions: In this review article, salivary biomarkers along with the circulating and tissue biomarkers were reviewed. Besides, some detection techniques were explained.
Keywords: Mouth, Neoplasm, Precancerous
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Irani S. Diagnostic strategies for early detection of oral squamous cell carcinoma: a review article. Avicenna J Dent Res. 2022; 14(1):137-143. doi:10.34172/ajdr.2022.25
Introduction
Oral squamous cell carcinoma (OSCC) is the sixth most common cancer and one of the main causes of cancer-related death around the world. Besides, oral cancer causes facial disfigurement and morbidity (1). The risk factors include smoking and alcohol consumption, chronic inflammation, UV radiation (lip cancer), HPV and Candida infections, immunosuppression, and genetic predisposition. Besides, oral microbiome and inflammatory cells play essential roles in the malignant transformation of the oral mucosa (2).Oral cancer patients are still diagnosed at advanced stages. Lymph node metastasis (especially to cervical lymph nodes) is an important prognostic factor and a leading cause for cancer-related death globally (3). Early diagnosis is an attractive strategy to improve survival rates in patients. Cancer control strategies depend on early diagnosis and detection which rely on clinical assessment. The delay in diagnosis can be attributed to either the patient or clinician (4). Total delay is a period of time from the patient’s first awareness of symptoms to the onset of treatment. In addition to patient’s awareness, clinical presentations can aid the clinician(5). Delayed diagnosis has mostly been indicated in the cases of gingival and buccal cancers (1). Screening programs such as mammography and Pap smear test are widely used for screening for breast cancer and cervical cancer; however, oral cancer is diagnosed only after progression of the disease. Therefore, conventional visual examination of the lesion under white light illumination and palpation play pivotal roles in the diagnosis of highly susceptible lesions. Based on current knowledge, tissue biopsy and histological examinations are the gold standards for accurate diagnosis of oral dysplasia at an early stage of disease progression. However, non-invasive biomarkers are required (6). According to the World Health Organization (WHO), lesions and conditions in the oral cavity, which may undergo malignant transformation, are referred to as oral potentially malignant disorders (OPMDs). It is proposed that 50% of oral cancers develop from precursor lesions. Hence, the early detection and proper management of precancerous lesions have great impacts on oral cancer prevention (7).
Methods
Search Study
A literature search in PubMed, ScienceDirect, and Google Scholar was performed. The keywords “early detection”, “oral cancer”, and “oral squamous cell carcinoma” were searched in the title and abstract of the articles published in English from 2000 to mid-2021. The full texts of 250 articles were retrieved and only 63 articles met the inclusion criteria. The inclusion criteria were the early detection, biomarkers, and detection techniques.
Results
Each article was carefullyread and analyzed. In summary, the selected papers discussed the importance of early detection and different factors and techniques to detect oral cancer. The biomarkers were divided into three groups as follows: salivary biomarkers, circulating biomarkers, and tissue biomarkers. Besides, some detection techniques are addressed below.
Oral Potentially Malignant Disorders (OPMDs)
OPPDs include leukoplakia, erythroplakia, erythroleukoplakia, proliferative verrucous leukoplakia, palatal reverse smoking, lichen planus, and submucous fibrosis (8,9). It is estimated that the global prevalence of OPMDs is 4.47%. However, the prevalence may vary between populations and genders. For example, it is higher in Asians and males (10). Tissue biopsy is the gold standard for cancer diagnosis. This technique is used for histological analysis and detection of early dysplastic changes. The degree of dysplastic changes in the epithelium is the main indicator of the risk of malignant transformation in OPMDs (11). Dysplasia is characterized by epithelial architectural disturbance and cellular atypia. Dysplasia is classified as mild, moderate, and severe according to the severity of epithelial involvement (12). Although histologic examination has been considered as the gold standard for the diagnosis of oral dysplastic and neoplastic changes, some other invasive or non-invasive techniques have been suggested as diagnostic tools.
Salivary Biomarkers as Non-invasive Diagnostic Tools
Saliva analysis is a noninvasive and inexpensive tool for cancer diagnosis. Compared to blood or tissue samples, saliva has a few advantages including the ease of collection, transport, and processing (13). Additionally, biomarkers in saliva are diluted and easily available. Importantly, saliva has a direct contact with oral cancer lesions, and hence is a suitable method for detecting oral cancer (14). Saliva is a biofluid that contains several proteins and factors, circulating and tissue derived cells, extracellular vesicles (EVs), DNA and RNA molecules, and different cytokines. A variety of salivary biomarkers can be detected in patients with OSCC such as cell-free DNAs (cfDNAs), circulating tumor DNA (ctDNA), EVs, and miRNAs. Necrotic and apoptotic cells can release DNA/RNA molecules into body fluids during physiologic and pathologic conditions (15). In physiological conditions, these molecules are phagocytic targets; however, they accumulate in the tissue microenvironment and biological fluids in cancers. It is believed that the increased number of apoptotic/necrotic cells in cancer patients is the underlying mechanism (15).
cfDNAs are short (70–200 bp) or long (up to 21 kb) fragments of double-stranded DNA. They can be detected in blood, saliva, plasma, urine, cerebrospinal fluid, and other bodily fluids. cfDNA is freely circulating DNA, but it does not necessarily originate from a tumor as it can be released from normal dying cells. Apoptotic tissue and hematological cells which release the DNA into the circulation are the major sources of cfDNA in a body fluid. In healthy individuals, cfDNA is found at low levels because apoptotic cells and cfDNA are cleared very quickly. However, in chronic inflammation and cancer cases, clearance is inadequate; therefore, cfDNA can accumulate. In cancer patients, cfDNAs are found in tissue samples and reflect the genetic and epigenetic alterations (16). Circulating tumor DNAs (ctDNAs) are tumor-derived fragmented DNAs which are not associated with cancer cells. Several cancer characteristics such as cellular turnover, vascularity, and drug responses are related to ctDNA concentrations. Although ctDNA is mainly released into the bloodstream, it can be found in other body fluids including saliva. Interestingly, due to less dilution and contamination, the analysis of ctDNA in saliva is highly sensitive (16).
miRNAs, small endogenous single-strand RNA molecules, are dysregulated in many diseases (17). miRNAs can control different events in cancers such as proliferation, differentiation, apoptosis, survival, motility, invasion, and metastasis (18). Salivary miRNAs are the potential biomarkers for detection of oral cancer (19). For instance, down-regulation of salivary miR-139-5p has been detected in early tongue cancer (20). In a previously published study, salivary miR-31 level was evaluated in patients with OSCC and patients with oral verrucous leukoplakia. According to the results of this study, miR-31 level was significantly increased in patients with oral carcinoma at all clinical stages, compared to that in patients with oral verrucous leukoplakia (21). A study carried out on saliva samples showed an elevated miR-21 expression level in the salivary samples of OLP, dysplastic OLP, and OSCC patients compared to those of control individuals. In addition, a significantly increased expression level of miR-31 was found in samples from dysplastic OLP and OSCC patients compared to those from healthy controls (22).
EVs are lipid bilayer-delimited particles that are naturally released from cells. EVs represent one of the intercellular communications found in tumor microenvironment (TME) and saliva. In saliva, cell-derived EVs contain some factors such as DNAs, RNAs, miRNAs, and proteins. Recent investigations have indicated that EVs may have essential roles in oral cancer growth. The most studied vesicles in tumor growth are micro-vesicles and exosomes (15). Exosomes are small membrane vesicles, ranging from 40–150 nm, which are present in the TME. Exosomes contain proteins, lipids, mRNAs, miRNAs, and mitochondrial DNA. They are released from a variety of cells into biological fluids such as saliva, urine, semen, amniotic fluid, cerebrospinal fluid, lymph, tears, and blood in physiologic and pathologic conditions. Salivary exosomal miRNAs are considered as diagnostic biomarkers for various malignancies, including OSCC (23). For example, salivary exosomal miR-24-3p has been demonstrated as a potential biomarker for OSCC (24). In addition, tumor-derived exosomes in saliva have divergent morphological and molecular characteristics. Therefore, they can be used in the early detection of precancerous lesions and malignant transformation (15). Exosomes also play essential roles in pro-metastatic niche formation, as well as bone marrow and lymph node metastases. Exosomes can be used as a predictive tool in cancer patients and to differentiate healthy individuals from cancer patients (14). It is suggested that exosomes contribute to tumorigenesis, invasion, and metastasis (25). Besides, salivary exosomes contain elevated IgA level which has a significant role in the local immune response of the oral cavity (26). Dysregulation of some other factors and cytokines can be found in saliva. For example, a published paper revealed increased levels of salivary tumor necrosis factor alpha (TNF-α), a cell signaling protein, in patients with OSCC compared to healthy control subjects and patients with leukoplakia. The authors suggested the salivary TNF-α level as a tool for monitoring the malignant transformation of leukoplakia to OSCC (27). An early investigation on salivary and serum IL-6 levels showed significant differences in oral premalignant lesions and oral cancer. IL-6 promotes cancer cell proliferation and involves in the inactivation of the p53 tumor suppressor gene (28). Some data have shown significant correlations between salivary and serum biomarkers. However, some others have shown that biomarkers may differ between blood and saliva. For instance, extracellular RNA biomarkers in the blood are different from those in the saliva. Standard techniques for saliva collection and processing criteria play essential roles in the reliability of the analysis (29).
Circulating Biomarkers
cfDNAs are released into the bloodstream. However, a detailed study which analyzed the plasma level of cfDNAs in 390 patients (90 potentially malignant lesions, 150 OSCCs, and 150 post-treatment OSCCs) and 150 healthy controls did not find any significant difference between the examined groups. The authors proposed that due to the rich lymphatic drainage of the oral mucosa, cfDNA does not enter the bloodstream (30).
Circulating DNA (ctDNA) can be detected in different forms such as free DNA, protein-bound complexes either as free circulating molecules or encapsulated in vesicles (apoptotic bodies, microvesicles, and exosomes). It is now clear that ctDNA has an active role in carcinogenesis. CtDNA has a short half-life of around 10 to 15 minutes. It is rapidly degraded by blood nucleases and eliminated by the liver, spleen, and kidneys (31).
Circulating miRNAs or cell-free miRNAs, a class of short non-coding RNAs, are also considered as noninvasive cancer biomarkers. Interestingly, cell-free miRNAs are encapsulated in lipoprotein complexes, and hence are protected from endogenous RNase activity in body fluids (32). Circulating miRNA levels have been studied in patients with oral premalignant lesions and OSCC. For instance, the expression level of miR-21 was assessed in the serum of patients with oral submucous fibrosis (OSF) and OSCC. According to the results of this study, a significant difference was found between OSF and OSCC patients in terms of miR-21 expression level. Additionally, there was a significant relationship between the expression of miR-21 and the clinical stages of OSCC patients (33). Data collected from a previous study has shown that up-regulation of plasma miR-10b can be used as an early detection marker for oral cancer (34). In a published study, circulating miR-196a and miR-196b were assessed in plasma samples of 53 healthy individuals, 16 pre-cancer patients, and 90 oral cancer patients. The results showed a significant distinction between normal and precancerous patients and between normal and cancer patients. The authors suggested that the combination of miR-196a and miR-196b may be a useful tool for the early detection of oral cancer (35). Circulating expression levels of 3 miRNAs (miR-222-3p, miR-423-5p, and miR-150-5p) were indicated in patients with oral leukoplakia and OSCC in a published article. According to the findings of this study, miR-222-3p expression level was significantly down-regulated in leukoplakia patients compared to the normal and OSCC patients whereas miR-423-5p and miR150-5p expression levels were elevated in OSCC patients compared to the healthy individuals and leukoplakic patients. The authors suggested miR-222-3p, miR-423-5p, and miR-150-5p as potential biomarkers for the early diagnosis of OSCC and oral leukoplakia (36).
A number of scientists have indicated that circulating tumor cells (CTCs) can also be used to diagnose cancer at an early stage. CTCs are rare epithelial cells shed from primary tumor into the vasculature. CTCs are found in 30%–40% of cancer patients. CTCs can be considered as prognostic markers (37). Morphologically, CTCs are similar to the primary solid tumor cells and play crucial roles in metastasis. Hence, it is important to identify patients with CTCs to predict metastasis at an early stage. CTCs have been detected in patients with head and neck SCC and correlate with lymph node metastasis (37). CTCs undergo epithelial-mesenchymal transition (EMT), a crucial event in cancer development, to migrate to distant organs (38,39). In a previous study on OSCC patients, CTCs were detected in 12.5% of patients with OSCC. Surprisingly, significant correlations were found between CTCs and tumor size (40). Circulating tumor microemboli (CTM) are clusters of tumor cells. It is proposed that CTM can be easily caught in narrow vessels than CTCs, which provide a new suitable environment for the survival of tumor cells (25,41).
Circulating cytokines are also involved in malignant transformation and tumor growth. The role of cytokines has been evaluated in oral premalignant lesions. According to the results of a study, cytokines such as TNF-a, TGF-β1, and IL-6 have significant effects on the risk of development of precancerous lesions (42).
Tissue Biomarkers
Aberrant levels of miRNAs have been detected in oral samples. For instance, the expression of miR-375 was significantly decreased after progression from premalignant lesion to OSCC. It is suggested that miR-375 expression level is a useful tool to identify progressive premalignant lesions from non-progressive samples (43). Recent studies have shown that OSCC parental cells release exosomes with oncogenic markers which are able to influence the surrounding TME. In OSCC, exosomes are present in TME and can increase the expression of TGF-β, a key player signaling pathway in tumor progression (44). TME consists of different cell types. Among them, cancer-associated fibroblasts (CAFs) have the capacity to transport miRNAs and proteins to cells by exosomes (45). CAF-derived exosomes enhance OSCC metastasis (46,47).
In a previous study on oral leukoplakia and OSCC samples, the expression level of p53 and epithelial growth factor receptor (EGFR) increased as the lesion progressed from non-dysplastic lesions to moderate dysplastic lesions. The authors proposed that p53 and EGFR play critical roles in the progression of premalignant lesions to carcinomas (48). Cytokeratins (CKs) are useful biomarkers for the assessment of histopathological progression of oral cancer. In a previously published paper, the expression of CK10-ab1 was assessed in keratinized squamous stratified epithelium. Sever expression of CK10-ab1 was indicated in the suprabasal layers of all specimens in normal and hyperplastic samples; however, CK10-ab1 disappeared gradually with the progression of malignant changes. Therefore, the expression of CK10-ab1 was mild in all poorly differentiated SCCs. The authors suggested CK10-ab1 as a predictable marker for the early detection of OSCC (49). A detailed investigation on CDK1 has demonstrated a higher expression level of CDK1 in OSCC samples. In this study, there was a significant correlation between the expression level of CDK1 and histological grade of OSCC. Hence, overexpression of CDK1 was found in high grade tumors (50). Moreover, cytokines and growth factors produced by inflammatory cells can be stained in tissue specimens. Increased expression levels of IL-1α, Il-1β, TGF-β, platelet-derived growth factor, and basic fibroblast growth factor have been found in both epithelium and underlying connective tissue of OSF samples (51). Besides, the expression of NF-κB has been detected in oral premalignant and malignant lesions. A previous study has reported a statistically significant gradual increase of NF-κΒ cytoplasmic immunostaining score from normal mucosa to OSCC (52). EMT markers can also be detected in oral potentially malignant disorders (OPMDs) s and cancer tissue samples. Expression levels of E-cadherin and vimentin, the most important EMT markers, were assessed in 64 OMPD tissue samples and 23 malignant cases of oral cavity using immunohistochemical (IHC). The results of this study showed a significantly reduced expression level of E-Cadherin in invasive carcinoma samples compared to dysplastic and carcinoma in situ cases. In this study, the expression level of vimentin was positively correlated with tumor progression (53). Additionally, early detection of OSCC can rely on the identification of new biomarkers of extracellular matrix such as matrix metalloproteinases (MMPs) (54). Higher expression levels of MMP-1 and MMP-9 have been demonstrated in OMPDs which progressed to cancer (55).Figure 1 represents a summary of the biomarkers which can be used for the early detection of OSCC.
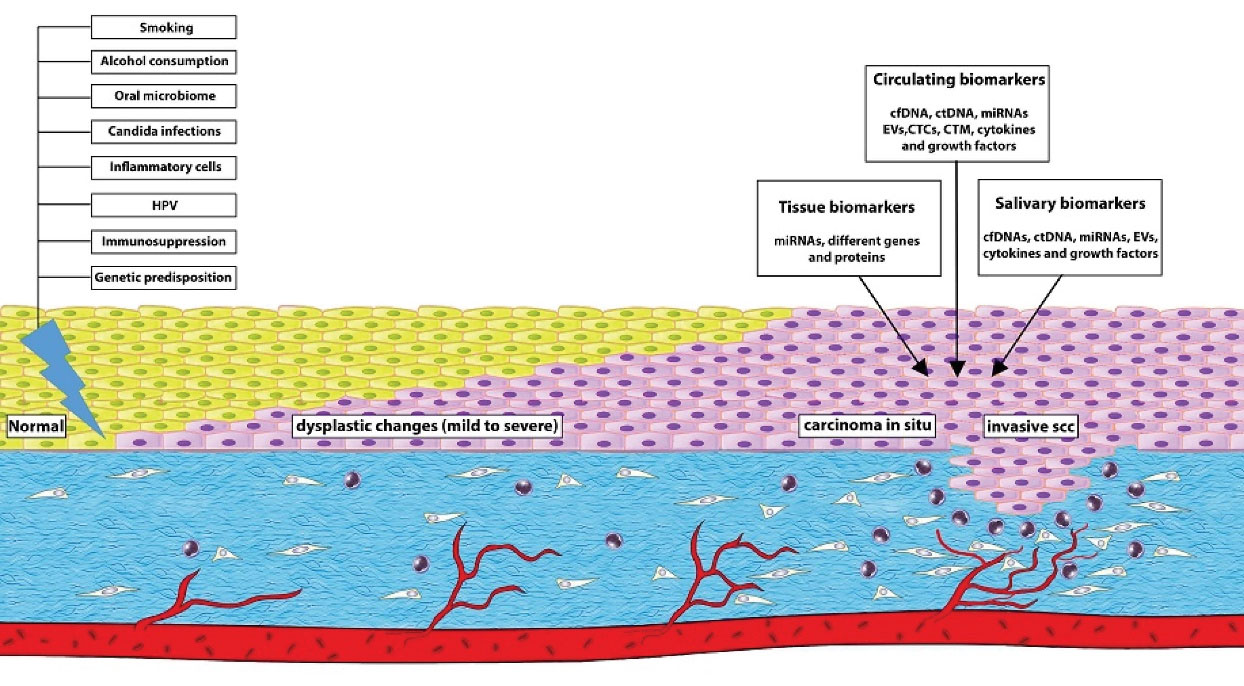
Figure 1.
Overview of the major risk factors which promote oral dysplasia and develop oral cancer. Molecular biomarkers can aid in the early detection, monitoring and prognosis of oral cancer.
.
Overview of the major risk factors which promote oral dysplasia and develop oral cancer. Molecular biomarkers can aid in the early detection, monitoring and prognosis of oral cancer.
Detection Techniques
As the early diagnosis of oral cancer is a key factor in improving the survival and quality of life, it is important to employ different detection techniques. In addition to tissue biopsy, some other helpful techniques are addressed below.
(A) Brush biopsy obtains specimens from all three layers; the basal, intermediate, and superficial layers. A brush biopsy is a non-invasive and painless technique that facilitates cytological analysis. However, the problem is that the diagnosis needs to be confirmed by tissue biopsy; therefore, it delays the diagnosis.
(B) The fluorescence method (chemiluminescence) is also used for the early detection of precancerous and cancerous lesions. It is the direct visualization of oral cavity and has a good sensitivity to detect oral precancerous lesions, but it only detects leukoplakia not erythroplakia. In addition, this technique costs quite high (6,56).
(C) Toluidine blue stains the cells with an increased amount of DNA and broader intercellular canals. A previously published study has indicated that the sensitivity and specificity of toluidine blue test are 92.6% and 67.9%, respectively, and the overall diagnostic accuracy is 80%. The toluidine blue staining is simple, rapid, and noninvasive. In addition, the application of toluidine blue can reduce the number of biopsies (57).
(D) The methylene blue staining shows the increased amount of DNA in potentially malignant cells. Accumulating data indicates that methylene blue has high sensitivity but low specificity (58).
(E) IHC uses antibodies to detect the location of proteins and other antigens in tissue samples. It is also easy to determine microvessel density by IHC (59).Occult nodal metastasis may be unnoticed in the routine pathological examination but serial sectioning and immunohistochemistry with pan-cytokeratin markers can help in the early detection of micro-metastasis (59).
(F) Liquid biopsy is a non-invasive method to detect OSCC. In contrast to tissue biopsy, liquid biopsy is easy, less invasive, and more comfortable for patients. Liquid biopsy is also a helpful method for detecting HPV DNA. A previously published study has found a higher level of HPV-16/18 DNA (cfDNA) in the plasma of 14% of patients (60).
(G) Proteomics, the large-scale study of proteins, investigates different proteins expressed in cells and tissues. A variety of tools can be used for proteomic analysis such as ELISA, PCR, Western Blot, and IHC (61).
(H) For a successful result, some other methods are also helpful. For example, Sanger sequencing, q-PCR-based methods, fluorescent assays, and chromatographic methods are powerful tools for cfDNA analysis (30). miRNAs can be detected by microarray analysis, RT-qPCR, northern blotting, and in situ hybridization. Recently, nanomaterials such as gold nanoparticles, magnetic nanoparticles, silver nanoclusters, and quantum dots have been applied for miRNA detection (62). CTCs can be detected by telomerase activity, aptamer technology, and the CELLSEARCH system (41). A variety of techniques based on microfluidics such as Lab-on-a-chip, microfluidic digital PCR, microfluidic single-cell RT-PCR, digital microfluidics, and microfluidic co-culture technique based on 3D spheroids are useful in analyzing genetic mutations, TME, cell proliferation, cancer growth, and interactions between cancer cells and mesenchymal cells (61). CTCs can be detected and isolated by telomerase activity, aptamer technology, the CELLSEARCH system, and microfluidic technologies (41,61).
Therapy
After the correct diagnosis of OPMDs, a regular follow-up by an oral health professional is recommended. All risk factors such as smoking and alcohol consumption should be controlled. High-risk lesions such as leukoplakias, erythroplakias, and erythroleukoplakias with moderate or severe dysplasia should be excised. Patient follow-up at appropriate intervals is highly recommended (10). Surgical treatments such as excision, cryosurgery, and carbon dioxide (CO2) laser ablation can be useful. Accumulating evidence shows that the surgically treated precancerous lesions cannot reduce the rate of malignant transformation. It is believed that field cancerization is the underlying mechanism (8). However, premalignant lesions can be managed only by observation and administration of chemopreventive compounds including retinoids, cyclooxygenase-2 inhibitors, epidermal growth factor receptor inhibitors/antagonists, and p53 modulators. The most extensively studied chemopreventive compounds are the retinoids, but they are limited to the treatment of oral leukoplakia (63). The awareness of public and clinicians of the risk factors and early signs of precancerous lesions plays a critical role in prevention. Preventative health care strategies are important to minimize the rate of morbidity and mortality from oral cancer (63).Prevention, screening techniques, and early detection can minimize morbidity and mortality rates. Experience and training of oral health practitioners are the best diagnostic techniques(63).
Conclusion
OPMDs are considered risk factors for the development of OSCC. Avoiding smoking and alcohol consumption can have beneficial effects on oral health. It is important to detect primary OSCC at an early clinical stage. It is needed to improve the dentists’ knowledge about the early detection of potentially malignant and oral cancer lesions by encouraging them to participate in cancer prevention programs. Such training programs could reduce delay in diagnosis. Besides, oral cancer awareness among the public should also be improved. In addition to clinical examination and histologic evaluation, some new techniques in the field of molecular and cellular biology are available to detect malignant transformation. When a persistent lesion is detected, a biopsy should be performed. After complete excision of the lesion, adequate follow-up and monitoring are required.
Ethical Statement
Not applicable.
Conflict of Interest Disclosures
None declared.
Funding
This review article has no funding source.
References
- Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol 2010; 46(6):414-7. doi: 10.1016/j.oraloncology.2010.03.009 [Crossref] [ Google Scholar]
- Irani S, Barati I, Badiei M. Periodontitis and oral cancer-current concepts of the etiopathogenesis. Oncol Rev 2020; 14(1):465. doi: 10.4081/oncol.2020.465 [Crossref] [ Google Scholar]
- Irani S. Metastasis to head and neck area: a 16-year retrospective study. Am J Otolaryngol 2011; 32(1):24-7. doi: 10.1016/j.amjoto.2009.09.006 [Crossref] [ Google Scholar]
- Sujir N, Ahmed J, Pai K, Denny C, Shenoy N. Challenges in early diagnosis of oral cancer: cases series. Acta Stomatol Croat 2019; 53(2):174-80. doi: 10.15644/asc53/2/10 [Crossref] [ Google Scholar]
- Gigliotti J, Madathil S, Makhoul N. Delays in oral cavity cancer. Int J Oral Maxillofac Surg 2019; 48(9):1131-7. doi: 10.1016/j.ijom.2019.02.015 [Crossref] [ Google Scholar]
- McRae MP, Modak SS, Simmons GW, Trochesset DA, Kerr AR, Thornhill MH. Point-of-care oral cytology tool for the screening and assessment of potentially malignant oral lesions. Cancer Cytopathol 2020; 128(3):207-20. doi: 10.1002/cncy.22236 [Crossref] [ Google Scholar]
- Starzyńska A, Pawłowska A, Renkielska D, Michajłowski I, Sobjanek M, Błażewicz I. Oral premalignant lesions: epidemiological and clinical analysis in the northern Polish population. Postepy Dermatol Alergol 2014; 31(6):341-50. doi: 10.5114/pdia.2014.40932 [Crossref] [ Google Scholar]
- Irani S. Pre-cancerous lesions in the oral and maxillofacial region: a literature review with special focus on etiopathogenesis. Iran J Pathol 2016; 11(4):303-22. [ Google Scholar]
- Irani S, Monsef Esfahani A, Ghorbani A. Dysplastic change rate in cases of oral lichen planus: a retrospective study of 112 cases in an Iranian population. J Oral Maxillofac Pathol 2016; 20(3):395-9. doi: 10.4103/0973-029x.190911 [Crossref] [ Google Scholar]
- Warnakulasuriya S. Oral potentially malignant disorders: a comprehensive review on clinical aspects and management. Oral Oncol 2020; 102:104550. doi: 10.1016/j.oraloncology.2019.104550 [Crossref] [ Google Scholar]
- Ranganathan K, Kavitha L. Oral epithelial dysplasia: classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J Oral Maxillofac Pathol 2019; 23(1):19-27. doi: 10.4103/jomfp.JOMFP_13_19 [Crossref] [ Google Scholar]
- Ilhan B, Epstein JB, Guneri P. Potentially premalignant disorder/lesion versus potentially premalignant patient: relevance in clinical care. Oral Oncol 2019; 92:57-8. doi: 10.1016/j.oraloncology.2019.03.009 [Crossref] [ Google Scholar]
- Wang X, Kaczor-Urbanowicz KE, Wong DT. Salivary biomarkers in cancer detection. Med Oncol 2017; 34(1):7. doi: 10.1007/s12032-016-0863-4 [Crossref] [ Google Scholar]
- Nair S, Tang KD, Kenny L, Punyadeera C. Salivary exosomes as potential biomarkers in cancer. Oral Oncol 2018; 84:31-40. doi: 10.1016/j.oraloncology.2018.07.001 [Crossref] [ Google Scholar]
- Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: current status and perspectives. Front Physiol 2019; 10:1476. doi: 10.3389/fphys.2019.01476 [Crossref] [ Google Scholar]
- Fici P. Cell-free DNA in the liquid biopsy context: role and differences between ctDNA and CTC marker in cancer management. Methods Mol Biol 2019; 1909:47-73. doi: 10.1007/978-1-4939-8973-7_4 [Crossref] [ Google Scholar]
- Irani S, Shokri G. The role of miR-143, miR-145, and miR-590 in expression levels of CD44 and vascular endothelial cadherin in oral squamous cell carcinoma. Middle East J Cancer 2019; 10(3):194-204. doi: 10.30476/mejc.2019.78667 [Crossref] [ Google Scholar]
- Maroof H, Irani S, Arianna A, Vider J, Gopalan V, Lam AK. Interactions of vascular endothelial growth factor and p53 with miR-195 in thyroid carcinoma: possible therapeutic targets in aggressive thyroid cancers. Curr Cancer Drug Targets 2019; 19(7):561-70. doi: 10.2174/1568009618666180628154727 [Crossref] [ Google Scholar]
- Yoshizawa JM, Wong DT. Salivary microRNAs and oral cancer detection. Methods Mol Biol 2013; 936:313-24. doi: 10.1007/978-1-62703-083-0_24 [Crossref] [ Google Scholar]
- Duz MB, Karatas OF, Guzel E, Turgut NF, Yilmaz M, Creighton CJ. Identification of miR-139-5p as a saliva biomarker for tongue squamous cell carcinoma: a pilot study. Cell Oncol (Dordr) 2016; 39(2):187-93. doi: 10.1007/s13402-015-0259-z [Crossref] [ Google Scholar]
- Liu CJ, Lin SC, Yang CC, Cheng HW, Chang KW. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck 2012; 34(2):219-24. doi: 10.1002/hed.21713 [Crossref] [ Google Scholar]
- Mehdipour M, Shahidi M, Manifar S, Jafari S, Mashhadi Abbas F, Barati M. Diagnostic and prognostic relevance of salivary microRNA-21, -125a, -31 and -200a levels in patients with oral lichen planus-a short report. Cell Oncol (Dordr) 2018; 41(3):329-34. doi: 10.1007/s13402-018-0372-x [Crossref] [ Google Scholar]
- Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int 2014; 2014:864894. doi: 10.1155/2014/864894 [Crossref] [ Google Scholar]
- He L, Ping F, Fan Z, Zhang C, Deng M, Cheng B. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed Pharmacother 2020; 121:109553. doi: 10.1016/j.biopha.2019.109553 [Crossref] [ Google Scholar]
- Irani S. Emerging insights into the biology of metastasis: a review article. Iran J Basic Med Sci 2019; 22(8):833-47. doi: 10.22038/ijbms.2019.32786.7839 [Crossref] [ Google Scholar]
- Cheshmi B, Cheshomi H. Salivary exosomes: properties, medical applications, and isolation methods. Mol Biol Rep 2020; 47(8):6295-307. doi: 10.1007/s11033-020-05659-1 [Crossref] [ Google Scholar]
- Deepthi G, Nandan SR, Kulkarni PG. Salivary tumour necrosis factor-α as a biomarker in oral leukoplakia and oral squamous cell carcinoma. Asian Pac J Cancer Prev 2019; 20(7):2087-93. doi: 10.31557/apjcp.2019.20.7.2087 [Crossref] [ Google Scholar]
- Dineshkumar T, Ashwini BK, Rameshkumar A, Rajashree P, Ramya R, Rajkumar K. Salivary and serum interleukin-6 levels in oral premalignant disorders and squamous cell carcinoma: diagnostic value and clinicopathologic correlations. Asian Pac J Cancer Prev 2016; 17(11):4899-906. doi: 10.22034/apjcp.2016.17.11.4899 [Crossref] [ Google Scholar]
- Oh SY, Kang SM, Kang SH, Lee HJ, Kwon TG, Kim JW. Potential salivary mRNA biomarkers for early detection of oral cancer. J Clin Med 2020; 9(1):243. doi: 10.3390/jcm9010243 [Crossref] [ Google Scholar]
- Babji D, Nayak R, Bhat K, Kotrashetti V. Cell-free tumor DNA: emerging reality in oral squamous cell carcinoma. J Oral Maxillofac Pathol 2019; 23(2):273-9. doi: 10.4103/jomfp.JOMFP_36_19 [Crossref] [ Google Scholar]
- Bronkhorst AJ, Ungerer V, Holdenrieder S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol Detect Quantif 2019; 17:100087. doi: 10.1016/j.bdq.2019.100087 [Crossref] [ Google Scholar]
- Zhao C, Sun X, Li L. Biogenesis and function of extracellular miRNAs. ExRNA 2019; 1(1):38. doi: 10.1186/s41544-019-0039-4 [Crossref] [ Google Scholar]
- Singh P, Srivastava AN, Sharma R, Mateen S, Shukla B, Singh A. Circulating microRNA-21 expression as a novel serum biomarker for oral sub-mucous fibrosis and oral squamous cell carcinoma. Asian Pac J Cancer Prev 2018; 19(4):1053-7. doi: 10.22034/apjcp.2018.19.4.1053 [Crossref] [ Google Scholar]
- Lu YC, Chen YJ, Wang HM, Tsai CY, Chen WH, Huang YC. Oncogenic function and early detection potential of miRNA-10b in oral cancer as identified by microRNA profiling. Cancer Prev Res (Phila) 2012; 5(4):665-74. doi: 10.1158/1940-6207.capr-11-0358 [Crossref] [ Google Scholar]
- Lu YC, Chang JT, Huang YC, Huang CC, Chen WH, Lee LY. Combined determination of circulating miR-196a and miR-196b levels produces high sensitivity and specificity for early detection of oral cancer. Clin Biochem 2015; 48(3):115-21. doi: 10.1016/j.clinbiochem.2014.11.020 [Crossref] [ Google Scholar]
- Chang YA, Weng SL, Yang SF, Chou CH, Huang WC, Tu SJ. A three-microRNA signature as a potential biomarker for the early detection of oral cancer. Int J Mol Sci 2018; 19(3):758. doi: 10.3390/ijms19030758 [Crossref] [ Google Scholar]
- Hristozova T, Konschak R, Stromberger C, Fusi A, Liu Z, Weichert W. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann Oncol 2011; 22(8):1878-85. doi: 10.1093/annonc/mdr130 [Crossref] [ Google Scholar]
- Polioudaki H, Agelaki S, Chiotaki R, Politaki E, Mavroudis D, Matikas A. Variable expression levels of keratin and vimentin reveal differential EMT status of circulating tumor cells and correlation with clinical characteristics and outcome of patients with metastatic breast cancer. BMC Cancer 2015; 15:399. doi: 10.1186/s12885-015-1386-7 [Crossref] [ Google Scholar]
- Irani S, Dehghan A. Expression of vascular endothelial-cadherin in mucoepidermoid carcinoma: role in cancer development. J Int Soc Prev Community Dent 2017; 7(6):301-7. doi: 10.4103/jispcd.JISPCD_323_17 [Crossref] [ Google Scholar]
- Gröbe A, Blessmann M, Hanken H, Friedrich RE, Schön G, Wikner J. Prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of patients with squamous cell carcinoma of the oral cavity. Clin Cancer Res 2014; 20(2):425-33. doi: 10.1158/1078-0432.ccr-13-1101 [Crossref] [ Google Scholar]
- Anitha N, Jimson S, Masthan KM, Jacobina JJ. Circulating tumor cells in oral squamous cell carcinoma-an enigma or reality?. J Pharm Bioallied Sci 2015; 7(Suppl 1):S173-5. doi: 10.4103/0975-7406.155893 [Crossref] [ Google Scholar]
- Hsu HJ, Yang YH, Shieh TY, Chen CH, Kao YH, Yang CF. Role of cytokine gene (interferon-γ, transforming growth factor-β1, tumor necrosis factor-α, interleukin-6, and interleukin-10) polymorphisms in the risk of oral precancerous lesions in Taiwanese. Kaohsiung J Med Sci 2014; 30(11):551-8. doi: 10.1016/j.kjms.2014.09.003 [Crossref] [ Google Scholar]
- Harrandah AM, Fitzpatrick SG, Smith MH, Wang D, Cohen DM, Chan EK. MicroRNA-375 as a biomarker for malignant transformation in oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 122(6):743-52. doi: 10.1016/j.oooo.2016.07.022 [Crossref] [ Google Scholar]
- Lousada-Fernandez F, Rapado-Gonzalez O, Lopez-Cedrun JL, Lopez-Lopez R, Muinelo-Romay L, Suarez-Cunqueiro MM. Liquid biopsy in oral cancer. Int J Mol Sci 2018; 19(6):1704. doi: 10.3390/ijms19061704 [Crossref] [ Google Scholar]
- Li YY, Tao YW, Gao S, Li P, Zheng JM, Zhang SE. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018; 36:209-20. doi: 10.1016/j.ebiom.2018.09.006 [Crossref] [ Google Scholar]
- Bovy N, Blomme B, Frères P, Dederen S, Nivelles O, Lion M. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget 2015; 6(12):10253-66. doi: 10.18632/oncotarget.3520 [Crossref] [ Google Scholar]
- Wang X, Wang X, Xu M, Sheng W. Effects of CAF-derived microRNA on tumor biology and clinical applications. Cancers (Basel) 2021; 13(13):3160. doi: 10.3390/cancers13133160 [Crossref] [ Google Scholar]
- Singla S, Singla G, Zaheer S, Rawat DS, Mandal AK. Expression of p53, epidermal growth factor receptor, c-erbB2 in oral leukoplakias and oral squamous cell carcinomas. J Cancer Res Ther 2018; 14(2):388-93. doi: 10.4103/0973-1482.191027 [Crossref] [ Google Scholar]
- Ali AA, Al-Jandan BA, Suresh CS. The importance of ctokeratins in the early detection of oral squamous cell carcinoma. J Oral Maxillofac Pathol 2018; 22(3):441. doi: 10.4103/jomfp.JOMFP_238_17 [Crossref] [ Google Scholar]
- Chen X, Zhang FH, Chen QE, Wang YY, Wang YL, He JC. The clinical significance of CDK1 expression in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal 2015; 20(1):e7-12. doi: 10.4317/medoral.19841 [Crossref] [ Google Scholar]
- Haque MF, Harris M, Meghji S, Barrett AW. Immunolocalization of cytokines and growth factors in oral submucous fibrosis. Cytokine 1998; 10(9):713-9. doi: 10.1006/cyto.1997.0342 [Crossref] [ Google Scholar]
- Kamperos G, Nikitakis N, Sfakianou A, Avgoustidis D, Sklavounou-Andrikopoulou A. Expression of NF-κB and IL-6 in oral precancerous and cancerous lesions: an immunohistochemical study. Med Oral Patol Oral Cir Bucal 2016; 21(1):e6-13. doi: 10.4317/medoral.20570 [Crossref] [ Google Scholar]
- Akhtar K, Ara A, Siddiqui SA, Sherwani RK. Transition of immunohistochemical expression of E-cadherin and vimentin from premalignant to malignant lesions of oral cavity and oropharynx. Oman Med J 2016; 31(3):165-9. doi: 10.5001/omj.2016.33 [Crossref] [ Google Scholar]
- Kumar KV, Hema KN. Extracellular matrix in invasion and metastasis of oral squamous cell carcinoma. J Oral Maxillofac Pathol 2019; 23(1):10-6. doi: 10.4103/jomfp.JOMFP_97_19 [Crossref] [ Google Scholar]
- Jordan RC, Macabeo-Ong M, Shiboski CH, Dekker N, Ginzinger DG, Wong DT. Overexpression of matrix metalloproteinase-1 and -9 mRNA is associated with progression of oral dysplasia to cancer. Clin Cancer Res 2004; 10(19):6460-5. doi: 10.1158/1078-0432.ccr-04-0656 [Crossref] [ Google Scholar]
- Cicciù M, Cervino G, Fiorillo L, D’Amico C, Oteri G, Troiano G. Early diagnosis on oral and potentially oral malignant lesions: a systematic review on the VELscope® fluorescence method. Dent J (Basel) 2019; 7(3):93. doi: 10.3390/dj7030093 [Crossref] [ Google Scholar]
- Vijayakumar V, Reghunathan D, Edacheriyan B, Mukundan A. Role of toluidine blue staining in suspicious lesions of oral cavity and oropharynx. Indian J Otolaryngol Head Neck Surg 2019; 71(Suppl 1):142-6. doi: 10.1007/s12070-017-1161-y [Crossref] [ Google Scholar]
- Lejoy A, Arpita R, Krishna B, Venkatesh N. Methylene blue as a diagnostic aid in the early detection of potentially malignant and malignant lesions of oral mucosa. Ethiop J Health Sci 2016; 26(3):201-8. doi: 10.4314/ejhs.v26i3.2 [Crossref] [ Google Scholar]
- Irani S, Salajegheh A, Gopalan V, Smith RA, Lam AK. Expression profile of endothelin 1 and its receptor endothelin receptor A in papillary thyroid carcinoma and their correlations with clinicopathologic characteristics. Ann Diagn Pathol 2014; 18(2):43-8. doi: 10.1016/j.anndiagpath.2013.11.001 [Crossref] [ Google Scholar]
- Mazurek AM, Rutkowski T, Fiszer-Kierzkowska A, Małusecka E, Składowski K. Assessment of the total cfDNA and HPV16/18 detection in plasma samples of head and neck squamous cell carcinoma patients. Oral Oncol 2016; 54:36-41. doi: 10.1016/j.oraloncology.2015.12.002 [Crossref] [ Google Scholar]
- Madhura MG, Rao RS, Patil S, Fageeh HN, Alhazmi A, Awan KH. Advanced diagnostic aids for oral cancer. Dis Mon 2020; 66(12):101034. doi: 10.1016/j.disamonth.2020.101034 [Crossref] [ Google Scholar]
- Ye J, Xu M, Tian X, Cai S, Zeng S. Research advances in the detection of miRNA. J Pharm Anal 2019; 9(4):217-26. doi: 10.1016/j.jpha.2019.05.004 [Crossref] [ Google Scholar]
- Irani S. New insights into oral cancer-risk factors and prevention: a review of literature. Int J Prev Med 2020; 11:202. doi: 10.4103/ijpvm.IJPVM_403_18 [Crossref] [ Google Scholar]