Avicenna J Dent Res. 14(1):25-32.
doi: 10.34172/ajdr.2022.05
Original Article
Identification of Potential Anti-tooth-decay Compounds From Organic Cinnamic Acid Derivatives by Inhibiting Matrix Metalloproteinase-8: An In Silico Study
Amir Taherkhani 1  , Athena Orangi 2
, Athena Orangi 2  , Shirin Moradkhani 3
, Shirin Moradkhani 3  , Alireza Jalalvand 4
, Alireza Jalalvand 4  , Zahra Khamverdi 2, *
, Zahra Khamverdi 2, * 
Author information:
1Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
2Dental Research Center, Department of Operative Dentistry, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran
3Department of Pharmacognosy, School of Pharmacy, Medicinal Plants and Natural Product Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
4Department of Influenza and Other Respiratory Viruses, Pasteur Institute of Iran, Tehran, Iran
*
Corresponding author: Zahra Khamverdi, Dental Research Center, Department of Operative Dentistry, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran, Fax:+98-8138381085, Phone:+98-9183122095, Email:
dr.zahra.khamverdi@gmail.com
Abstract
Background: Matrix metalloproteinase-8 (MMP-8) is the most abundant member of the MMP family in human dentin. It takes a part in the normal physiology of tissue remodeling and wound healing, while the overexpression/hyperactivity of this protein leads to several oral disorders, including dental caries and peri-implant inflammation/diseases, and therefore, MMP-8 inhibition may have therapeutic effects. Accordingly, the current study aimed to identify potential MMP-8 inhibitors from cinnamic acid derivatives.
Methods: The binding affinity of cinnamic acid and its several derivatives to the MMP-8 active site were estimated using the AutoDock 4.0 software. The pharmacokinetics, toxicity, and bioavailability of top-ranked MMP-8 inhibitors were also predicted by utilizing bioinformatics web tools.
Results: Five of the studied components, including chlorogenic acid (CGA), caffeic acid 3-glucoside, rosmarinic acid, N-p-Coumaroyltyramine, and caffeic acid phenethyl ester (CAPE) demonstrated a salient affinity of binding to the MMP-8 catalytic site (∆Gbinding<-10 kcal/mol). It was estimated that these compounds can inhibit the MMP-8 at the nanomolar concentration, and therefore, were considered as top-ranked MMP-8 inhibitors. Finally, none of the top-ranked components revealed a considerable side effect and thus were found to be suitable for oral use.
Conclusions: The results of the present study suggested that CGA, caffeic acid 3-glucoside, rosmarinic acid, N-p-coumaroyltyramine, and CAPE might have protective effects on tooth decay and peri-implant inflammation/diseases.
Keywords: Cinnamic acid, Inhibitor, Matrix metalloproteinase-8, Molecular docking, Tooth caries, Tooth decay
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Taherkhani A, Orangi A, Moradkhani S, Jalalvand A, Khamverdi Z. Identification of potential anti-tooth-decay compounds from organic cinnamic acid derivatives by inhibiting matrix metalloproteinase-8: an in silico study. Avicenna J Dent Res. 2022; 14(1):25-32. doi:10.34172/ajdr.2022.05
Background
Matrix metalloproteinases (MMPs) are one of the most important enzymes that play a significant role in the normal physiology of cells (e.g., tissue remodeling and wound healing) and the etiology of several diseases. They are zinc- and calcium-dependent enzymes and are classified into several groups based on their substrates, including collagenases, gelatinases, stromelysins, stromelysins, matrilysins, member-type, and other types of MMPs that have not undergone classification. Neutrophils are the sources of MMP-8, and therefore, they have also been named neutrophil collagenase or collagenase-2 (1-3). Previous studies have indicated that in periodontal and peri-implant inflammation/diseases, the active form of MMP-8 was elevated in oral fluids (4-6). Moreover, the overexpression of MMP-8 has also been demonstrated in the oral cavity of patients with Crohn’s disease (7,8), as well as the saliva of patients suffering from caries lesions (9). Accordingly, the inhibition of MMP-8 may have preventive/therapeutic effects on several oral diseases.
Cinnamic acids are organic compounds with a basic structure of C6-C3, named phenylpropanoid backbone, and are mostly found in herbs and microorganisms (10). Cinnamic acid is the principal component that is found in many plants such as Cinnamomum cassia, and Panax ginseng, as well as vegetables, grains, and honey (11). It is derived from phenylalanine and has several pharmaceutical advantages, including antioxidant, anti-inflammatory, anticancer, and antibacterial properties (12-14). Additionally, cinnamic acid can result in several derivatives with many beneficial effects, including anti-inflammatory, antimicrobial (15), antidiabetic (16), and anticancer (17) activities. Several previous studies (18-20) have experimentally confirmed the anti-bacterial effects of cinnamic acid and its several derivatives on Streptococcus mutans andPorphyromonas gingivalis. S. mutans and P. gingivalis are well known as the main pathogens that are responsible for the initiation/progression of dental caries and periodontitis, respectively (20-22). Therefore, the biological efficacy of cinnamic acid and its derivatives have been considered for scientists regarding designing/discovering drug candidates for therapeutic aims in various disorders (23). In the present study, it was suggested that cinnamic acid and its derivatives may be effective compounds in the inhibition of MMP-8. Thus, this study was designed based on molecular docking simulations to examine the binding affinity of cinnamic acid and its several derivatives to the catalytic site of the MMP-8.
Materials and Methods
Structural Preparation and Molecular Docking
The structure of the MMP-8 and the ligands tested in the current study, including cinnamic acid and a total of 11 cinnamic acid derivatives, were downloaded from the Structural Bioinformatics database (https://www.rcsb.org) and the PubChem database (https://pubchem.ncbi.nlm.nih.gov), respectively (24,25). The Protein Data Bank file with the ID of 4QKZ contained the three-dimensional structure of MMP-8, as well as the inhibitor of the MMP-8 (named QZK) in the Pochetti et al study with the criteria of X-ray resolution of 1.2 Å (https://www.rcsb.org/structure/4QKZ). Energy optimization was applied before molecular docking simulations for MMP-8 and all ligands. All docking operations were performed by utilizing the AutoDock software, version 4.0 (http://autodock.scripps.edu) (26). The AutoDock estimates the binding energy (∆Gbinding) between the ligand and the receptor using the Lamarckian genetic algorithm. The catalytic site of the MMP-8 was considered a docking pocket. The details of energy optimization, grid box options, and the residues identified within the catalytic domain of the MMP-8 are reported in our previous study (27).
As shown in Figure 1, cinnamic acid is an organic aromatic carboxylic acid (11) with several pharmaceutical characteristics, including antioxidant, antimicrobial (13), anti-inflammatory, antidiabetic (28), and anticancer effects (14). Although this acid could be synthesized by the enzymatic deamination of phenylalanine (29), it is naturally produced in herbs (30). Several derivatives of cinnamic acid are achieved by the modification of the benzene ring and the acrylic acid group (12,23,31). In this study, several features were considered for ligand selection from cinnamic acid derivatives. To this end, being a herb was the main character because of its low side effect and high availability (32). In addition, demonstrating antibacterial effects against tooth caries-related bacteria in previous studies was considered as another important feature of the components.
Figure 1.
Chemical Structure of Cinnamic Acid Achieved by the ChemDraw (version 12.0.2.1076).
.
Chemical Structure of Cinnamic Acid Achieved by the ChemDraw (version 12.0.2.1076).
Drug-likeness Study
The Rule of Five (RO5), which has been presented by Lipinski et al (33), was considered to predict the drug-likeness of the tested compounds in the present study using the PubChem database. According to the RO5, the orally administered drugs must confirm at least three of the incoming physical/chemical properties (Mass ≤ 500 g/mol, Log of the partition coefficient between octanol and water (LogP) ≤ 5, number of accepting H-bonds ≤ 10, and number of the H-bond donor ≤ 5).
Absorption, Distribution, Metabolism, Excretion, and Toxicity
The absorption, distribution, metabolism, excretion (ADME), in addition to the toxicity (ADMET) of the top-ranked inhibitors, were taken into consideration by applying SwissADME (http://www.swissadme.ch/) and the PreADMET (https://preadmet.bmdrc.kr/) webservers. The carcinogenicity of the compounds in rats and mice and the possible inhibitory effect of the components on the human ether-a-go-go-related gene channel of the heart were predicted to evaluate the toxicity of the ligands. Several pharmacokinetic characteristics of the components were referred to the ADME, including the gastrointestinal absorption, blood-brain barrier permeability, possible inhibition of the cytochrome P-450, and possible substrate for the P-glycoprotein. SwissADME applies several vigorous algorithms such as support vector machine, the Ward method, and a reciprocal nearest neighbor algorithm to achieve more reliable results (34).
Results
Affinity of Binding Between the MMP-8 and Small Molecules
Among 12 ligands tested in the present study, a total of five compounds revealed a salient binding affinity to the MMP-8 catalytic site with the criteria of ∆Gbinding ≤ -10 kcal/mol, including chlorogenic acid (CGA), caffeic acid 3-glucoside, rosmarinic acid, N-p-coumaroyltyramine, and caffeic acid phenethyl ester (CAPE). Therefore, these cinnamic acid derivatives were considered as top-ranked MMP-8 inhibitors. The inhibition constant (Ki) value for these components was predicted to be at the nanomolar (nM) concentration. According to our previous research (27), the binding affinity and the Ki value for the control component (QZK) were estimated to be -9.45 kcal/mol and 118.80 nM. Hence, the results of the molecular docking analysis represented that the affinity of binding between the top-ranked cinnamic acid derivatives, as well as the cynarin, and the MMP-8 catalytic domain is more than that of QZK. Table 1 presents the ∆Gbinding and the Ki value of all components evaluated in this study. Figure 2 illustrates the binding affinity between cinnamic acid and its derivatives, the control inhibitor, and the MMP-8 catalytic site. For post-docking analysis, the interaction modes between top-ranked MM-P8 inhibitors and the residues within the catalytic site of the MMP-8 were screened by utilizing the BIOVIA Discovery Studio Visualizer 19.1.0.18287 (https://discover.3ds.com/discovery-studio-visualizer-download). Table 2 and Figure 3 demonstrate these interactions as a table and figure, respectively. Figure 4 depicts all the interactions between top-rank cinnamic acid derivatives and their corresponding residues in a unique graph by the Cytoscape software (https://cytoscape.org/download.html) (35). Figure 5 illustrates the number of interactions calculated for each top-ranked MMP-8 inhibitor called a degree.
Table 1.
The Binding Affinity to the MMP-8 Catalytic Site and the Ki Value Estimated for Cinnamic Acid and its Derivatives as Compared With the Control Component (QZK)
|
PubChem ID
|
Ligand Name
|
Estimated Energy of Binding (kcal/mol)
|
K
i |
| 1 794 427 |
Chlorogenic acid |
-11.81 |
2.22 nM |
| 5 281 759 |
Caffeic acid 3-glucoside |
-11.26 |
5.57 nM |
| 5 281 792 |
Rosmarinic acid |
-11.03 |
8.20 nM |
| 5 372 945 |
N-p-Coumaroyltyramine |
-10.25 |
30.78 nM |
| 5 281 787 |
Caffeic acid phenethyl ester |
-10.23 |
31.98 nM |
| 6 124 212 |
Cynarin |
-9.58 |
94.45 nM |
| 637 540 |
o-Coumaric acid |
-7.37 |
3.95 uM |
| 689 043 |
Caffeic acid |
-7.12 |
6.07 uM |
| 445 858 |
Ferulic acid |
-6.80 |
10.40 uM |
| 637 542 |
p-Coumaric acid |
-6.44 |
18.98 uM |
| 444 539 |
Cinnamic acid |
-6.11 |
33.28 uM |
| 637 775 |
Sinapinic acid |
-5.95 |
43.21 uM |
| 53 361 485 |
QZK (Ctrl) |
-9.45 |
118.80 nM |
Note. Ki: Inhibition constant; Ctrl: Control.
Table 2.
Interaction Modes Identified Between Top-ranked Cinnamic Acid Derivatives and the Residues Within the MMP-8 Catalytic Site
|
Ligand Name
|
Hydrogen Bond (Distance Å)
|
Hydrophobic Interaction (Distance Å)
|
Unfavorable (Distance Å)
|
| Chlorogenic acid |
Leu160 (3.13); Asn218 (5.28) |
His197 (4.08); Val194 (3.97); Tyr219 (4.67) |
Ala220 (3.44) |
| Caffeic acid 3-glucoside |
Glu198 (4.80); Pro217 (4.57); Ala213 (5.43); Leu214 (3.55); Ala220 (3.70); Gly158 (4.19); Ala161 (4.94) |
His197 (4.25); Val194 (5.48) |
Ala220 (3.70) |
| Rosmarinic acid |
Tyr219 (4.28); Ala220 (3.01) |
His197 (4.46); Ile159 (5.86) |
NA |
| N-p-Coumaroyltyramine |
Ala220 (3.62, 6.17); Pro217 (5.31) |
His197 (3.90, 4.14); Tyr219 (4.85) |
NA |
| Caffeic acid phenethyl ester |
Tyr219 (6.11); Pro217 (6.00); Asn218 (4.60) |
His197 (4.04) |
NA |
Note. MMP-8: Matrix metalloproteinase-8; NA: Not available.
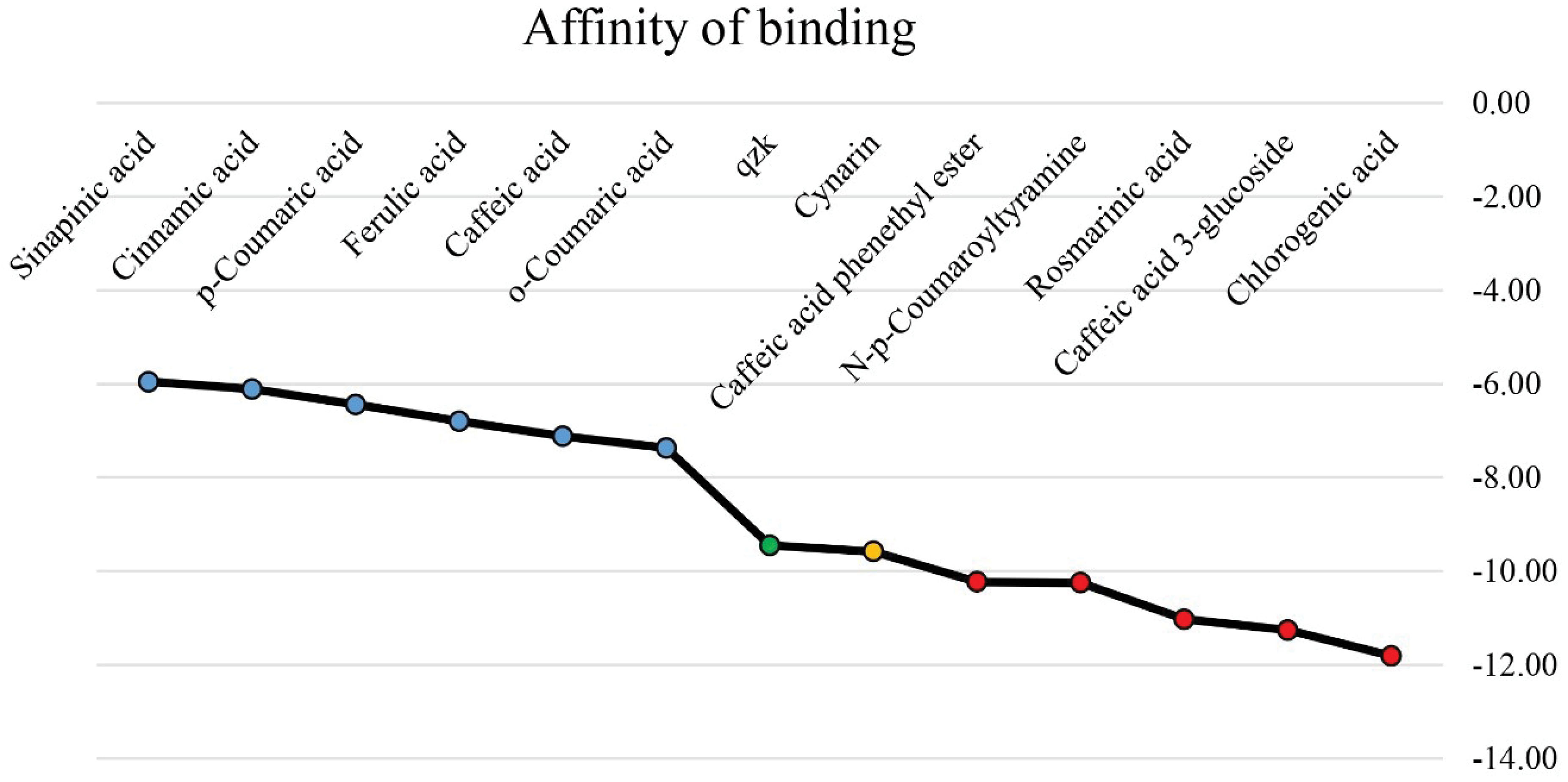
Figure 2.
The Binding Affinity of Cinnamic Acid and its Derivatives to the Catalytic Domain of MMP-8 Compared to the Control Component (QZK). Note. X-axis demonstrates the name of the ligands. The green dot illustrates the MMP-8 control inhibitor (QZK). Red circles show the top-ranked MMP-8 inhibitors with the ∆G binding ≤ -10 kcal/mol, and the orange spot represents cynarin with the estimated energy of binding more negative than the QZK. In addition, the blue ones demonstrate the compounds with lower binding affinity to the MMP-8 catalytic site as compared with the QZK. Y-axis depicts the estimated energy of binding (kcal/mol).
.
The Binding Affinity of Cinnamic Acid and its Derivatives to the Catalytic Domain of MMP-8 Compared to the Control Component (QZK). Note. X-axis demonstrates the name of the ligands. The green dot illustrates the MMP-8 control inhibitor (QZK). Red circles show the top-ranked MMP-8 inhibitors with the ∆G binding ≤ -10 kcal/mol, and the orange spot represents cynarin with the estimated energy of binding more negative than the QZK. In addition, the blue ones demonstrate the compounds with lower binding affinity to the MMP-8 catalytic site as compared with the QZK. Y-axis depicts the estimated energy of binding (kcal/mol).
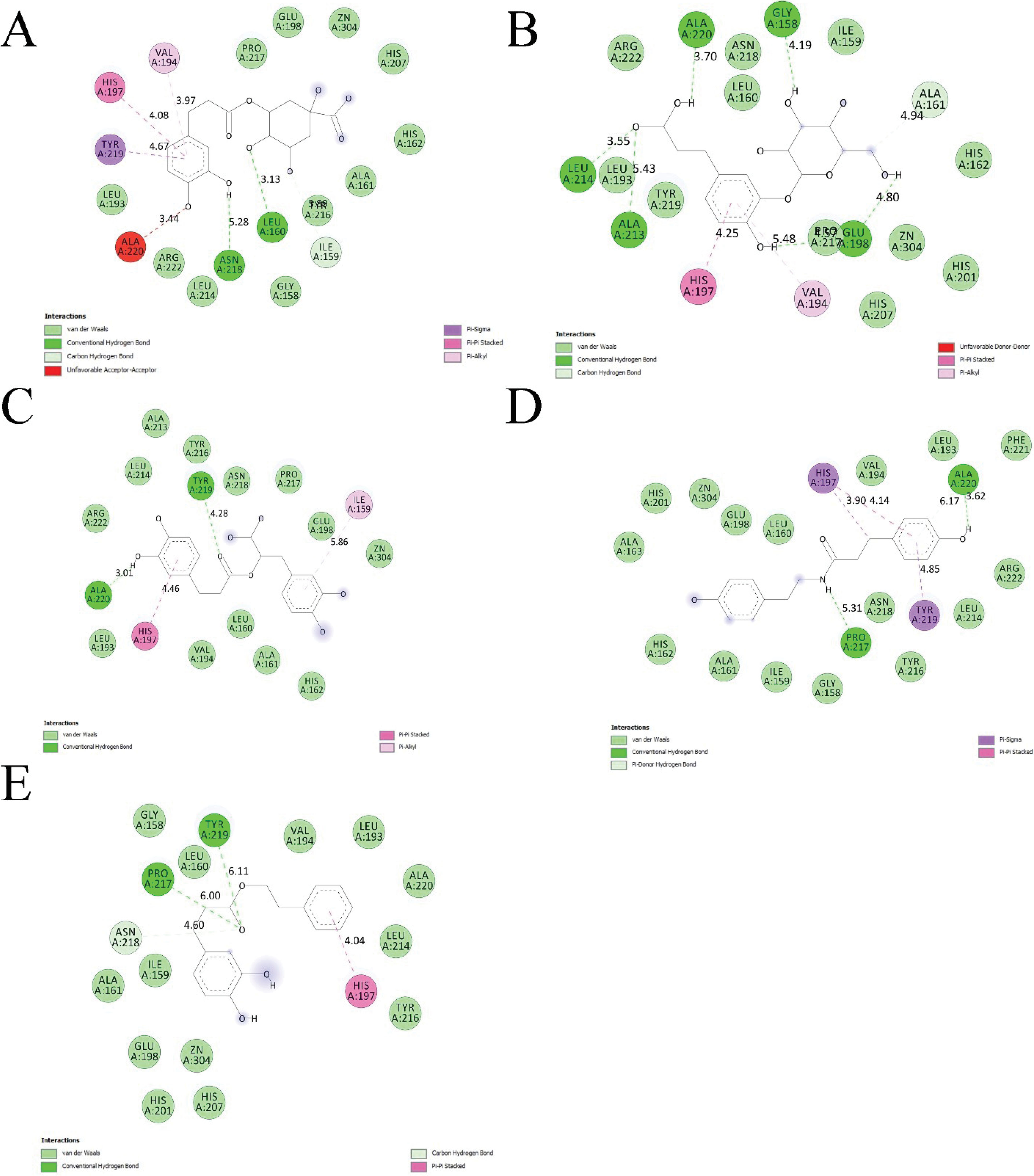
Figure 3.
Interaction Types Detected Between Residues Inside the MMP-8 Catalytic Domain and (A) Chlorogenic Acid, (B) Caffeic Acid 3-glucoside, (C) Rosmarinic Acid, (D) N-p-Coumaroyltyramine, and (E) Caffeic Acid Phenethyl Ester After the Post-Docking Analysis.
.
Interaction Types Detected Between Residues Inside the MMP-8 Catalytic Domain and (A) Chlorogenic Acid, (B) Caffeic Acid 3-glucoside, (C) Rosmarinic Acid, (D) N-p-Coumaroyltyramine, and (E) Caffeic Acid Phenethyl Ester After the Post-Docking Analysis.
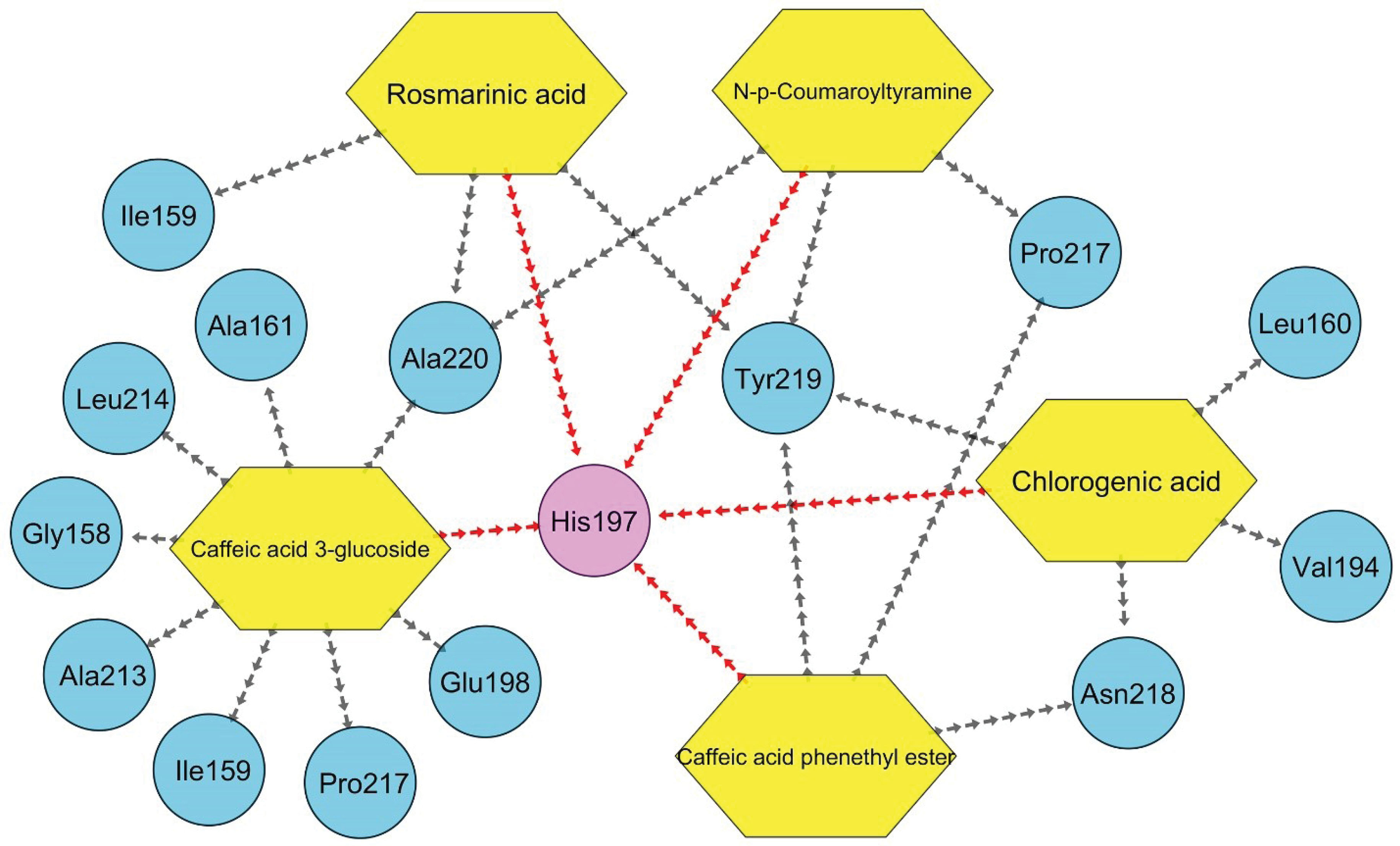
Figure 4.
A Unique Graph Demonstrating All Interactions Between Top-ranked Compounds and Their Corresponding Amino Acids Within the MMP-8 Catalytic Site. Note. The red lines show a π-π paring interaction, which is known as one of the most stabilizing interactions between the ligand and the receptor.
.
A Unique Graph Demonstrating All Interactions Between Top-ranked Compounds and Their Corresponding Amino Acids Within the MMP-8 Catalytic Site. Note. The red lines show a π-π paring interaction, which is known as one of the most stabilizing interactions between the ligand and the receptor.
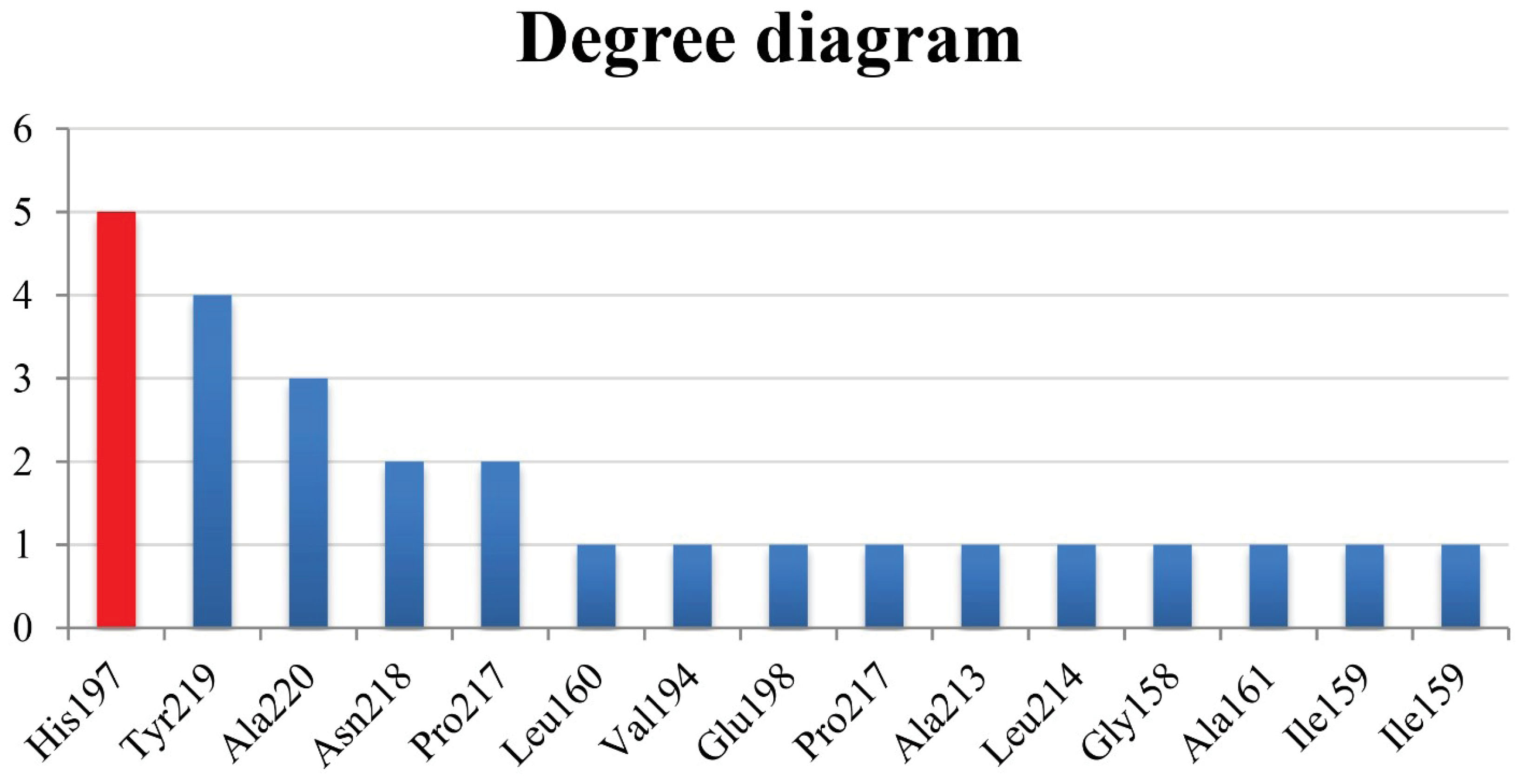
Figure 5.
Degree Chart. Note. X and y axes represent the residues and their corresponding degree, respectively.
.
Degree Chart. Note. X and y axes represent the residues and their corresponding degree, respectively.
Bioavailability of Top-ranked Compounds
All chemical and physical characteristics of the top-ranked MMP-8 inhibitors were analyzed based on the RO5. Interestingly, all of them were found to agree with Lipinski’s law, and therefore, CGA, caffeic acid 3-glucoside, rosmarinic acid, N-p-coumaroyltyramine, and CAPE were confirmed to be suitable for oral use (Table 3).
Table 3.
Chemo-physical Characteristics of the Top-ranked MMP-8 Inhibitors
|
Ligand Name
|
Molecular Weight (g/mol)
|
LogP
|
Hydrogen Bond Donor Count
|
Hydrogen Bond Acceptor Count
|
Orally Active Drug
|
| Chlorogenic acid |
354.31 |
-0.4 |
6 |
9 |
Yes |
| Caffeic acid 3-glucoside |
342.3 |
-1.4 |
6 |
9 |
Yes |
| Rosmarinic acid |
360.3 |
2.4 |
5 |
8 |
Yes |
| N-p-Coumaroyltyramine |
283.32 |
2.7 |
3 |
3 |
Yes |
| Caffeic acid phenethyl ester |
284.31 |
4.2 |
2 |
4 |
Yes |
Note. LogP: The logarithm of the partition coefficient between n-octanol and water.
Pharmacokinetics and Toxicity of Top-ranked Compounds
The ADMET prediction study revealed no considerable toxicity for the top-ranked cinnamic acid derivatives. However, CGA and caffeic acid 3-glucoside were found to be safer than the other top-ranked compounds. Furthermore, N-p-Coumaroyltyramine and CAPE showed higher gastrointestinal absorbance compared to other compounds (Table 4).
Table 4.
Pharmacokinetics and Toxicity of Top-Ranked Cinnamic Acid Derivatives Predicted Using Bioinformatics Webservers
|
Ligand Name
|
ADMET
|
Toxicity
|
|
GI abs
|
BBB Permeant
|
P-gp Substrate
|
CYP1A2 Inhibitor
|
CYP2C19 Inhibitor
|
CYP2C9 Inhibitor
|
CYP2D6 Inhibitor
|
CYP3A4 Inhibitor
|
hERG Inhibition
|
Carcino_mous
|
Carcino_rat
|
| Chlorogenic acid |
Low |
No |
No |
No |
No |
No |
No |
No |
Medium risk |
Negative |
Negative |
| Caffeic acid 3-glucoside |
Low |
No |
No |
No |
No |
No |
No |
No |
Medium risk |
Negative |
Negative |
| Rosmarinic acid |
Low |
No |
No |
No |
No |
No |
No |
No |
Medium risk |
Negative |
Positive |
| N-p-Coumaroyltyramine |
High |
Yes |
No |
No |
No |
No |
Yes |
Yes |
Medium risk |
Negative |
Negative |
Caffeic acid
phenethyl ester |
High |
Yes |
No |
Yes |
No |
No |
No |
No |
Medium risk |
Negative |
Negative |
Note. GI: Gastrointestinal; Abs: Absorption; BBB: Blood–brain barrier; P-gp: p-glycoprotein; CYP; Cytochrome p-450; Kp: Skin permeation coefficient; LD50: Lethal dose 50%; hERG: Human ether-a-go-go-related gene; ADMET: Absorption, distribution, metabolism, excretion toxicity.
Discussion
The enhanced expression and/or activity of MMP-8 is associated with several human disorders, including oral cavity and peri-implant inflammation/diseases. In the present study, molecular docking analysis was conducted to estimate the binding affinity of several natural compounds to the MMP-8 catalytic domain from cinnamic acid and its derivatives to discover drug candidates for MMP-8 inhibition.
Ribeiro et al (18) studied the anti-bacterial effects of several plant-based compounds, including cinnamic acid against S. mutans in vitro by indicating the minimum bacterial concentration for S. mutans.Based on their report, cinnamic acid revealed anti-microbial activity against S. mutans with low cytotoxic properties, suggesting that this compound may be useful for therapeutic aims of tooth decay. In the current study, it was estimated that cinnamic acid can connect to the MMP-8 catalytic domain with a ∆Gbinding of -6.11 kcal/mol, implying that cinnamic acid has a moderate affinity of binding to MMP-8. However, five of the cinnamic acid derivatives represented considerable binding affinity to the MMP-8 active site with the criteria of ∆Gbinding less than -10 kcal/mol, including CGA, caffeic acid 3-glucoside, rosmarinic acid, N-p-coumaroyltyramine, and CAPE.
CGA is a secondary metabolite in herbs with several pharmaceutical properties (e.g., antioxidant, antibacterial, cardioprotective, neuroprotective, and anti-inflammatory characteristics). Moreover, CGA can go through the bacteria cell and release the components of the cytoplasm, leading to bacteria death (36-38). Therefore, this compound has been widely used for tooth decay prevention. Palaniraj et al (19) examined the possible anti-biofilm effects of CGA when loaded to calcium phosphate-chitosan nanoparticles in restorative dentistry and reported that CGA significantly enhanced biofilm degradation up to 68%. Likewise, CGA revealed no toxicity effect on HaCaT cells up to 40 μg/mL. In the present study, CGA showed a considerable binding affinity to the MMP-8 catalytic domain with the ∆Gbinding of -11.81 kcal/mol. It was also estimated that this compound can inhibit the MMP-8 at the nanomolar scale Ki = 2.22 nM. CGA demonstrated three hydrophobic and two hydrogen bonds with Leu160, Val194, His197, Asn218, and Tyr219 within the MMP-8 catalytic domain. According to the potential inhibitory effect of CGA on MMP-8, in addition to the anti-bacterial activity of this component, CGA might be considered as a useful compound in restorative dentistry with protective effects against dental caries.
Similarly, Yamamoto and Ogawa (39) investigated the antimicrobial activity of perilla seed extracts against several bacteria involved in the pathogenesis of tooth caries and periodontitis, including oral streptococci and different strains of P. gingivalis (20). They concluded that rosmarinic acid revealed stronger antibacterial activity against various strains of P. gingivalis compared with oral streptococci. According to our results, rosmarinic acid can potentially connect to the MMP-8 catalytic site with a noticeable ∆Gbinding and Ki of -11.03 kcal/mol and 8 nM, respectively. Rosmarinic acid demonstrated two hydrogen and two hydrophobic interactions with Ile159, His197, Tyr219, and Ala220 within the MMP-8 active site. It may be concluded that rosmarinic acid has several protective effects on dental caries and periodontitis. However, confirmation is needed in this regard.
In another study, Kuramoto et al (40) found that CAPE significantly enhanced the expression and/or activity of the vascular endothelial growth factor (VEGF), nuclear factor-kappa B (NF-κB) transcription factor, and VEGF receptor- (VEGFR-) 2 in rat odontoblast cells (KN-3 cells), leading to elevated mineralization activity in KN-3 cells. Based on our findings, CAPE demonstrated a salient binding affinity to the MMP-8 catalytic domain with a ∆Gbinding of -10.23 kcal/mol. CAPE formed three hydrogen interactions and one hydrophobic interaction with His197, Pro217, Asn218, and Tyr219 inside the MMP-8 catalytic site. According to the findings of previous research, in addition to our results, it may be declared that CAPE could be considered as a new organic compound with conservative and regenerative properties in dental pulpal tissue as well as anti-tooth caries effects by inhibiting the MMP-8, and therefore, CAPE might be a useful compound in restorative dentistry (40). It is noteworthy that propolis is a rich source of CAPE (41,42). Further, the ∆Gbinding and Ki for caffeic acid 3-glucoside were evaluated to be -11.26 kcal/mol and 5.57 nM, suggesting a considerable affinity of binding between caffeic acid 3-glucoside and the MMP-8 active site. Caffeic acid 3-glucoside formed seven hydrogen and two hydrophobic interactions with Gly158, Ala161, Val194, His197, Glu198, Ala213, Leu214, Pro217, and Ala220 inside the MMP-8 catalytic domain.
N-p-Coumaroyltyramine is mainly found in Tribulus terrestris, which has been widely used in Chinese and Indian traditional medicine with several biological effects such as anticancer, antidiabetic, hepatoprotective, and anti-cariogenic properties (43). In addition, Oh et al (44) demonstrated that T. terrestris diminishes the caries-associated S. mutans. However, it should be identified that which of the active compounds within the T. terrestris is responsible for the inhibition of S. mutans. Based on our simulations, it was estimated that N-p-Coumaroyltyramine can inhibit the MMP-8 at the nanomolar scale (Ki = 30.78 nM) with the ∆Gbinding of -10.25 kcal/mol, representing that N-p-Coumaroyltyramine could be considered as an anti-tooth caries compound via inhibiting the normal activity of MMP-8. N-p-Coumaroyltyramine revealed three hydrophobic and three hydrogen interactions with the His197, Pro217, Tyr219, and Ala220 of the MMP-8 catalytic site.
It is worth mentioning that a π-π stacking hydrophobic interaction was detected between His197 and CGA (4.08 Å), caffeic acid 3-glucoside (4.25 Å), rosmarinic acid (4.46 Å), N-p-Coumaroyltyramine (4.14 Å), and CAPE (4.04 Å), proposing that these components can potentially form a stable connection with the MMP-8 catalytic site.
Conclusions
In general, it was estimated that five of the cinnamic acid derivatives, including CGA, caffeic acid 3-glucoside, rosmarinic acid, N-p-Coumaroyltyramine, and CAPE, can connect to the MMP-8 catalytic site at the nanomolar concentration with the criteria of ∆Gbinding < -10 kcal/mol, and therefore, were introduced as potential MMP-8 effective inhibitors. Additionally, these components all agreed with Lipinski’s RO5 and represented no significant toxicity, and thus may be beneficial for preventive/therapeutic aims in dentistry. Eventually, His197 was found to be the most active residue within the MMP-8 catalytic site. However, validation is inevitable in the future.
Acknowledgments
The authors would like to appreciate the Deputy of Research and Technology, Dental Research Center, and Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan-Iran for their support.
Authors’ Contributions
AT and ZK designed the study. Docking simulations were executed by AO and AT. Further, ADME and toxicity studies were performed by AO and AT. All images were processed by AO and AT. The results were analyzed and discussed by AT, ZK, AO, SM, and AJ. AT wrote the manuscript. All authors read and approved the final version of the manuscript.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflict of Interests Disclosures
The authors declare that they have no competing interests.
Ethics Statement
The current study was approved by the Ethics Committee of Hamadan University of Medical Sciences, Hamadan, Iran (ethics no. IR.UMSHA.REC.1398.576).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Kinane DF. Regulators of tissue destruction and homeostasis as diagnostic aids in periodontology. Periodontol 2000 2000; 24:215-25. doi: 10.1034/j.1600-0757.2000.2240110.x [Crossref] [ Google Scholar]
- Al-Majid A, Alassiri S, Rathnayake N, Tervahartiala T, Gieselmann DR, Sorsa T. Matrix metalloproteinase-8 as an inflammatory and prevention biomarker in periodontal and peri-implant diseases. Int J Dent 2018; 2018:7891323. doi: 10.1155/2018/7891323 [Crossref] [ Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006; 69(3):562-73. doi: 10.1016/j.cardiores.2005.12.002 [Crossref] [ Google Scholar]
- Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 2006; 38(5):306-21. doi: 10.1080/07853890600800103 [Crossref] [ Google Scholar]
- Sorsa T, Hernández M, Leppilahti J, Munjal S, Netuschil L, Mäntylä P. Detection of gingival crevicular fluid MMP-8 levels with different laboratory and chair-side methods. Oral Dis 2010; 16(1):39-45. doi: 10.1111/j.1601-0825.2009.01603.x [Crossref] [ Google Scholar]
- Sorsa T, Gursoy UK, Nwhator S, Hernandez M, Tervahartiala T, Leppilahti J. Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000 2016; 70(1):142-63. doi: 10.1111/prd.12101 [Crossref] [ Google Scholar]
- Schmidt J, Weigert M, Leuschner C, Hartmann H, Raddatz D, Haak R. Active matrix metalloproteinase-8 and periodontal bacteria-interlink between periodontitis and inflammatory bowel disease?. J Periodontol 2018; 89(6):699-707. doi: 10.1002/jper.17-0486 [Crossref] [ Google Scholar]
- Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Abdul Roda M. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut 2014; 63(4):578-87. doi: 10.1136/gutjnl-2012-303252 [Crossref] [ Google Scholar]
- Hedenbjörk-Lager A, Bjørndal L, Gustafsson A, Sorsa T, Tjäderhane L, Åkerman S. Caries correlates strongly to salivary levels of matrix metalloproteinase-8. Caries Res 2015; 49(1):1-8. doi: 10.1159/000360625 [Crossref] [ Google Scholar]
- Zolfaghari B, Yazdiniapour Z, Sadeghi M, Akbari M, Troiano R, Lanzotti V. Cinnamic acid derivatives from welsh onion (Allium fistulosum) and their antibacterial and cytotoxic activities. Phytochem Anal 2021; 32(1):84-90. doi: 10.1002/pca.2924 [Crossref] [ Google Scholar]
- Chandra S, Roy A, Jana M, Pahan K. Cinnamic acid activates PPARα to stimulate Lysosomal biogenesis and lower Amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol Dis 2019; 124:379-95. doi: 10.1016/j.nbd.2018.12.007 [Crossref] [ Google Scholar]
- Ruwizhi N, Aderibigbe BA. Cinnamic acid derivatives and their biological efficacy. Int J Mol Sci 2020; 21(16):5712. doi: 10.3390/ijms21165712 [Crossref] [ Google Scholar]
- Abd El-Raouf OM, El-Sayed EM, Manie MF. Cinnamic acid and cinnamaldehyde ameliorate cisplatin-induced splenotoxicity in rats. J Biochem Mol Toxicol 2015; 29(9):426-31. doi: 10.1002/jbt.21715 [Crossref] [ Google Scholar]
- Wang R, Yang W, Fan Y, Dehaen W, Li Y, Li H. Design and synthesis of the novel oleanolic acid-cinnamic acid ester derivatives and glycyrrhetinic acid-cinnamic acid ester derivatives with cytotoxic properties. Bioorg Chem 2019; 88:102951. doi: 10.1016/j.bioorg.2019.102951 [Crossref] [ Google Scholar]
- de Almeida Lima GD, Rodrigues MP, de Oliveira Mendes TA, Moreira GA, Siqueira RP, da Silva AM. Synthesis and antimetastatic activity evaluation of cinnamic acid derivatives containing 1,2,3-triazolic portions. Toxicol In Vitro 2018; 53:1-9. doi: 10.1016/j.tiv.2018.07.015 [Crossref] [ Google Scholar]
- Adisakwattana S, Pongsuwan J, Wungcharoen C, Yibchok-anun S. In vitro effects of cinnamic acid derivatives on protein tyrosine phosphatase 1B. J Enzyme Inhib Med Chem 2013; 28(5):1067-72. doi: 10.3109/14756366.2012.715286 [Crossref] [ Google Scholar]
- Gießel JM, Loesche A, Hoenke S, Csuk R. In search of new cinnamic acid derived flavours and fragrances. Results Chem 2019; 1:100010. doi: 10.1016/j.rechem.2019.100010 [Crossref] [ Google Scholar]
- Ribeiro M, Malheiro J, Grenho L, Fernandes MH, Simões M. Cytotoxicity and antimicrobial action of selected phytochemicals against planktonic and sessile Streptococcus mutans. PeerJ 2018; 6:e4872. doi: 10.7717/peerj.4872 [Crossref] [ Google Scholar]
- Palaniraj S, Murugesan R, Narayan S. Chlorogenic acid- loaded calcium phosphate chitosan nanogel as biofilm degradative materials. Int J Biochem Cell Biol 2019; 114:105566. doi: 10.1016/j.biocel.2019.105566 [Crossref] [ Google Scholar]
- Petit MD, van Steenbergen TJ, Timmerman MF, de Graaff J, van der Velden U. Prevalence of periodontitis and suspected periodontal pathogens in families of adult periodontitis patients. J Clin Periodontol 1994; 21(2):76-85. doi: 10.1111/j.1600-051x.1994.tb00283.x [Crossref] [ Google Scholar]
- Alaluusua S, Renkonen OV. Streptococcus mutans establishment and dental caries experience in children from 2 to 4 years old. Scand J Dent Res 1983; 91(6):453-7. doi: 10.1111/j.1600-0722.1983.tb00845.x [Crossref] [ Google Scholar]
- Singhrao SK, Harding A, Poole S, Kesavalu L, Crean S. Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s disease. Mediators Inflamm 2015; 2015:137357. doi: 10.1155/2015/137357 [Crossref] [ Google Scholar]
- Malheiro JF, Maillard JY, Borges F, Simões M. Evaluation of cinnamaldehyde and cinnamic acid derivatives in microbial growth control. Int Biodeterior Biodegradation 2019; 141:71-8. doi: 10.1016/j.ibiod.2018.06.003 [Crossref] [ Google Scholar]
- Deshpande N, Addess KJ, Bluhm WF, Merino-Ott JC, Townsend-Merino W, Zhang Q. The RCSB Protein Data Bank: a redesigned query system and relational database based on the mmCIF schema. Nucleic Acids Res 2005; 33(Database issue):D233-7. doi: 10.1093/nar/gki057 [Crossref] [ Google Scholar]
- Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A. PubChem substance and compound databases. Nucleic Acids Res 2016; 44(D1):D1202-13. doi: 10.1093/nar/gkv951 [Crossref] [ Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 2009; 30(16):2785-91. doi: 10.1002/jcc.21256 [Crossref] [ Google Scholar]
- Taherkhani A, Orangi A, Moradkhani S, Khamverdi Z. Molecular docking analysis of flavonoid compounds with matrix metalloproteinase-8 for the identification of potential effective inhibitors. Lett Drug Des Discov 2021; 18(1):16-45. doi: 10.2174/1570180817999200831094703 [Crossref] [ Google Scholar]
- Guo S, Zhen Y, Zhu Z, Zhou G, Zheng X. Cinnamic acid rescues behavioral deficits in a mouse model of traumatic brain injury by targeting miR-455-3p/HDAC2. Life Sci 2019; 235:116819. doi: 10.1016/j.lfs.2019.116819 [Crossref] [ Google Scholar]
- Li J, Li C, Smith SM. Hormone Metabolism and Signaling in Plants. Academic Press; 2017.
- Yilmaz S, Sova M, Ergün S. Antimicrobial activity of trans-cinnamic acid and commonly used antibiotics against important fish pathogens and nonpathogenic isolates. J Appl Microbiol 2018; 125(6):1714-27. doi: 10.1111/jam.14097 [Crossref] [ Google Scholar]
- Zhang WX, Wang H, Cui HR, Guo WB, Zhou F, Cai DS. Design, synthesis and biological evaluation of cinnamic acid derivatives with synergetic neuroprotection and angiogenesis effect. Eur J Med Chem 2019; 183:111695. doi: 10.1016/j.ejmech.2019.111695 [Crossref] [ Google Scholar]
- Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev 2010; 6(4):247-54. doi: 10.2174/157339910791658826 [Crossref] [ Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2001; 46(1-3):3-26. doi: 10.1016/s0169-409x(00)00129-0 [Crossref] [ Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 2017; 7:42717. doi: 10.1038/srep42717 [Crossref] [ Google Scholar]
- Saito R, Smoot ME, Ono K, Ruscheinski J, Wang PL, Lotia S. A travel guide to Cytoscape plugins. Nat Methods 2012; 9(11):1069-76. doi: 10.1038/nmeth.2212 [Crossref] [ Google Scholar]
- Lou Z, Wang H, Zhu S, Ma C, Wang Z. Antibacterial activity and mechanism of action of chlorogenic acid. J Food Sci 2011; 76(6):M398-403. doi: 10.1111/j.1750-3841.2011.02213.x [Crossref] [ Google Scholar]
- Petti S, Scully C. Polyphenols, oral health and disease: a review. J Dent 2009; 37(6):413-23. doi: 10.1016/j.jdent.2009.02.003 [Crossref] [ Google Scholar]
- Nallamuthu I, Devi A, Khanum F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J Pharm Sci 2015; 10(3):203-11. doi: 10.1016/j.ajps.2014.09.005 [Crossref] [ Google Scholar]
- Yamamoto H, Ogawa T. Antimicrobial activity of perilla seed polyphenols against oral pathogenic bacteria. Biosci Biotechnol Biochem 2002; 66(4):921-4. doi: 10.1271/bbb.66.921 [Crossref] [ Google Scholar]
- Kuramoto H, Hirao K, Yumoto H, Hosokawa Y, Nakanishi T, Takegawa D. Caffeic Acid Phenethyl Ester (CAPE) induces VEGF expression and production in rat odontoblastic cells. Biomed Res Int 2019; 2019:5390720. doi: 10.1155/2019/5390720 [Crossref] [ Google Scholar]
- Kasote DM, Pawar MV, Bhatia RS, Nandre VS, Gundu SS, Jagtap SD. HPLC, NMR based chemical profiling and biological characterisation of Indian propolis. Fitoterapia 2017; 122:52-60. doi: 10.1016/j.fitote.2017.08.011 [Crossref] [ Google Scholar]
- Georgieva K, Trusheva B, Uzunova V, Stoyanova T, Valcheva V, Popova M. New cycloartane triterpenes from bioactive extract of propolis from Pitcairn Island. Fitoterapia 2018; 128:233-41. doi: 10.1016/j.fitote.2018.05.024 [Crossref] [ Google Scholar]
- Chhatre S, Nesari T, Somani G, Kanchan D, Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn Rev 2014; 8(15):45-51. doi: 10.4103/0973-7847.125530 [Crossref] [ Google Scholar]
- Oh HK, Park SJ, Moon HD, Jun SH, Choi NY, You YO. Tribulus terrestris inhibits caries-inducing properties of Streptococcus mutans. J Med Plant Res 2011; 5(25):6061-6. doi: 10.5897/jmpr.9001270 [Crossref] [ Google Scholar]