Avicenna J Dent Res. 13(3):97-101.
doi: 10.34172/ajdr.2021.19
Original Article
Comparison of Sorption and Solubility Behavior of Four Different Types of Glass Ionomer Luting Cements in Artificial Saliva
Arash Shishehian 1, Fatemeh Amiri 1, *  , Alireza Izadi 1, Samaneh Abbasi 1, Maryam Farhadian 2, Armaqan Shahbazi 3
, Alireza Izadi 1, Samaneh Abbasi 1, Maryam Farhadian 2, Armaqan Shahbazi 3
Author information:
1Department of Prosthodontics, School of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran.
2Department of Health, School of Health, Hamadan University of Medical Sciences, Hamadan, Iran.
3Department of Prosthodontics, School of Dentistry, Tehran Medical Sciences, Islamic Azad Dentistry, Tehran, Iran.
Abstract
Background: Luting cement provides the connection between crowns and tooth structure. The sensitivity, solubility, and decomposition stages of the cement after the hardening stage are still subjects of relative controversy. These characteristics could lead to a poor connection between the braces and the teeth, increased probability of decay, and decalcification. The present study aimed to evaluate the adsorption and solubility of 4 types of glass ionomer cement.
Methods: Four luting cements were examined. A total of 10 specimens were prepared for each material following the manufacturer’s instructions, and the sorption and solubility were measured in accordance with the ISO 4049’s. Specimens were immersed in artificial saliva for 30 days, and were evaluated for sorption and solubility by first weighting them before incubation (W1), then immersing them in artificial saliva, dehydrating them in an oven for 24 hours, and weighing them again (W2 and W3, respectively). The data were analyzed using SPSS software version 21. One-way analysis of variance (ANOVA) followed by Tukey post hoc test was used to examine the differences among groups (α = 0.05).
Results: As for the both sorption and solubility, there was a significant interaction between the sorption and solubility of all materials (P<0.001). The sorption values in artificial saliva were highest for glass ionomer cement Riva Luting followed by GC Fuji 1 and Cavex, whereas the least value was observed for Meron (P<0.000). As for solubility, it was significantly higher in Cavex followed by GC Fuji1 and Meron, but it was significantly lower in Riva Luting.
Conclusions: It was determined that the weight changes of glass ionomer cements significantly varied among all the materials. Riva Luting followed by GC Fuji 1 had the highest water sorption, and the solubility was significantly higher in Cavex followed by GC Fuji1. Meron improved both water sorption and solubility properties among all glass ionomer cements.
Keywords: Glass ionomer cements, Sorption and solubility, Cement, Artificial saliva
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Shishehian A, Amiri F, Izadi A, Abbasi S, Farhadian M, Shahbazi A. comparison of sorption and solubility behavior of four different types of glass ionomer luting cements in artificial saliva. Avicenna J Dent Res. 2021;13(3):97-101. doi: 10.34172/ajdr.2021.19.
Background
Highlights
Glass-ionomer cements belong to the acid-base class of cements. They are formed as the result of a reaction between weak polymeric acids and powdered glasses, and are commonly used as an aqueous solution of polymeric acid and a glass powder, which are mixed by an appropriate method to form a viscous paste that sets rapidly (1). Glass-ionomers have various applications; for example, they can be used as restorative materials especially in the primary dentition, liners and bases, fissure sealants, as well as bonding agents for orthodontic brackets. Regarding the biological, physical, and chemical properties of glass ionomer cements, they are considered as the most appropriate restorative materials for atraumatic restorative treatment. Since these materials display longer settling time and more desirable physical-mechanical properties compared to the previous cements, they provide a higher survival rate of the restorations (2).
The clinical success and durability of luting cements in the oral cavity depend on the structural integrity and dimensional stability which are the results of water sorption and solubility. When cements are exposed to the oral cavity, their monomers could be dissolved and their solubility could be measured (3). Solubility refers to weight loss of per volume unit due to the decomposition of a material during the time it is exposed to saliva or oral fluids (4). Water sorption is among the negative characteristics of dental materials that lead to degradation of the cement, debonding of the restoration, and recurrent decay. It causes an increase in the volume of dental material, which may attenuate the mechanical properties of cement-tooth bond and cause subsequent saliva leakage and microorganisms to the cement-tooth interface (5). Therefore, cement water sorption and solubility may produce unfavorable outcomes including degradation of the cement leading to fracture of the restoration, marginal leakage, and the risk of secondary decay (6). Absorbed water acts as a plasticizer and leads to degradation of the filler-matrix interface, and solubility produces toxic substances such as formaldehyde and methacrylic acid. Accumulation of these products along with the residual monomers and fillers can lead to unpleasant consequences (6,7).
The clinical success of the atraumatic restorative treatments for restorations depends on the shelf-life of the restorative material (8). Wise choice of new products as restorative materials requires sufficient knowledge about physical and mechanical properties of the products; moreover, the solubility and sorption of various glass ionomer cements have not been widely investigated so far. This in vitro study, therefore, aimed to compare the sorption and solubility of four glass ionomer cements (Meron, Cavex, Gc Fuji1, Riva Luting) by using artificial water or saliva as a storage solution and by making an attempt to simulate the condition found in oral cavity.
Materials and Methods
Table 1 contains the descriptions of all luting cements used in this study. For each luting cement, a total of 10 bar-shaped specimens were prepared. for assessing the water solubility and sorption (Table 1). Cement bars of the four luting cements (Meron, Cavex, Gc Fuji1, Riva Luting) were prepared using the molds. For each material, 10 bar-shaped specimens 6±0.1 mm in height and 4±0.1 mm in thickness were employed. The molds were filled with powder/liquid ratios according to the manufacturer’s recommendations. Then they were filled with the materials and pressed using two glass plates under hand pressure to extrude any excess material.
Table 1.
Description of All the Cements Used in This Study
|
Cement
|
Manufacturer
|
Type
|
Mixing Time
|
Powder/Liquid
|
Expiry Date
|
Batch#
|
| Meron |
Voco, Germany |
Glass Ionomer |
30 s |
3:1 |
2023 |
1923313 |
| Cavex |
Cavex, the Netherlands |
Glass Ionomer |
30 s |
3:1 |
2024 |
1827193 |
| GC Fuji1 |
GC Corp |
Glass Ionomer |
20 s |
1:8:1 |
2023 |
180425c |
| Riva Luting |
SDI, Germany, GmbH |
Glass Ionomer |
10 s |
1:8:1 |
2024 |
11194643 |
Evaluation of Sorption and Solubility in Artificial Saliva
The initial weight (W1 (μg)) of all samples was measured to an accuracy of 0.0001 g using an analytical weighing scale (Ohaus Corporation; New Jersey, 07058, USA). Then, the samples were immersed into artificial saliva with a pH of 7 and stored at 37°C for 30 days. After the given time period, the specimens were removed, washed with water, dried with an absorbent paper, and weighed as W2 (μg). The samples were then dehydrated in an oven at 37°C for 24 hours and weighed again as W3 (μg).
The diameter(a), thickness(b) and height(h) of each specimen were measured at two points at right angles to each other, and the mean diameter,thickness calculated.. The volume (V) of each specimen was calculated as follows in cubic millimeters:
V = a × b × h
The loss of material (solubility) was obtained by examining the difference between the initial and final drying mass of each sample (W1-W3).
The amount of sorption was determined by comparing the difference between the weight of the samples after immersion in saliva and their final weight after removing them from the oven (W2--W3).
The values of sorption (Wsorp) and solubility (Wsol) were calculated using the following equations (ISO 4049: 2000):
Wsp = (W2–W3)/V
Wsol = (W1–W3)/V
where V is the volume of sample in mm3.
The data were statistically analyzed by SPSS software (version 21; SPSS Inc., Chicago, IL, USA). Analysis of the sorption and solubility values was performed using one-way analysis of variance (ANOVA) and post-hoc Tukey test. All statistical analyses were conducted at a significance level of P< 0.05.
Results
The normality of solubility distribution and sorption for all different groups was evaluated and confirmed by Kolmogorov-Smirnov test (P > 0.05).
One-way ANOVA technique was adopted to compare the mean solubility and sorption in different cements (α = 0.05).
The means and standard deviations for sorption and solubility are shown in Table 2. For both sorption and solubility, one-way ANOVA followed by Tukey test showed a significant interaction between the sorption and solubility of all materials (P< 0.05). Therefore, it was discovered that the effects of saliva on the materials differed in degree. The sorption values in artificial saliva were highest for Riva Luting followed by GC Fuji 1 and Cavex, and the least value was recorded for Meron (P< 0.000). As for solubility, significant interactions were observed between the materials and saliva (P< 0.000). The solubility was significantly higher in Cavex followed by GC Fuji1 and Meron, but it was significantly lower in Riva Luting.
Table 2.
Mean Sorption and Solubility Values (µg/mm)±(SD) of Four Glass Ionomer Cements in Artificial Saliva Using Tukey’s Test
|
Variable
|
Cement
|
N
|
Mean
|
Standard Deviation
|
95% Confidence Interval for Mean
|
Minimum
|
Maximum
|
|
Lower Bound
|
Upper Bound
|
| Sorption |
Meron |
10 |
296/65 |
17/29 |
284/28 |
309/02 |
273/36 |
330/49 |
| Cavex |
10 |
373/43 |
16/36 |
361/72 |
385/13 |
350/88 |
395/88 |
| GC Fuji1 |
10 |
471/23 |
34/59 |
446/49 |
495/98 |
416/67 |
518/00 |
| Riva Luting |
10 |
527/98 |
31/64 |
505/35 |
550/61 |
474/24 |
581/17 |
| Solubility |
Meron |
10 |
98/96 |
8/74 |
92/70 |
105/21 |
84/84 |
115/74 |
| Cavex |
10 |
191/39 |
24/22 |
174/06 |
208/71 |
157/77 |
233/03 |
| GC Fuji1 |
10 |
103/28 |
22/87 |
86/92 |
119/64 |
76/46 |
144/39 |
| Riva Luting |
10 |
36/12 |
16/55 |
24/28 |
47/95 |
12/48 |
64/21 |
The results from the analysis of variance regarding the comparison of sorption and solubility in different cements showed that there were significant differences between groups in terms of sorption (P < 0.001) and solubility (P < 0.001). The results from the analysis of variance are shown in Table 3.
Table 3.
Analysis of Variance
|
|
Sum of squares
|
Mean Square
|
P
value
|
Sorption Between Group
Within Groups |
316396.387
24875.485 |
105465.462
690.986 |
.000 |
Solubility Between Group
Within Groups |
122236.883
13139.245 |
40745.628
364.979 |
.000 |
Due to the significance of the variance test analysis, Tukey post-hoc test was used to compare different cements.
A box plot for comparing solubility in different groups is given in Figure 1. A box plot for comparing sorption in different groups is given in Figure 2.
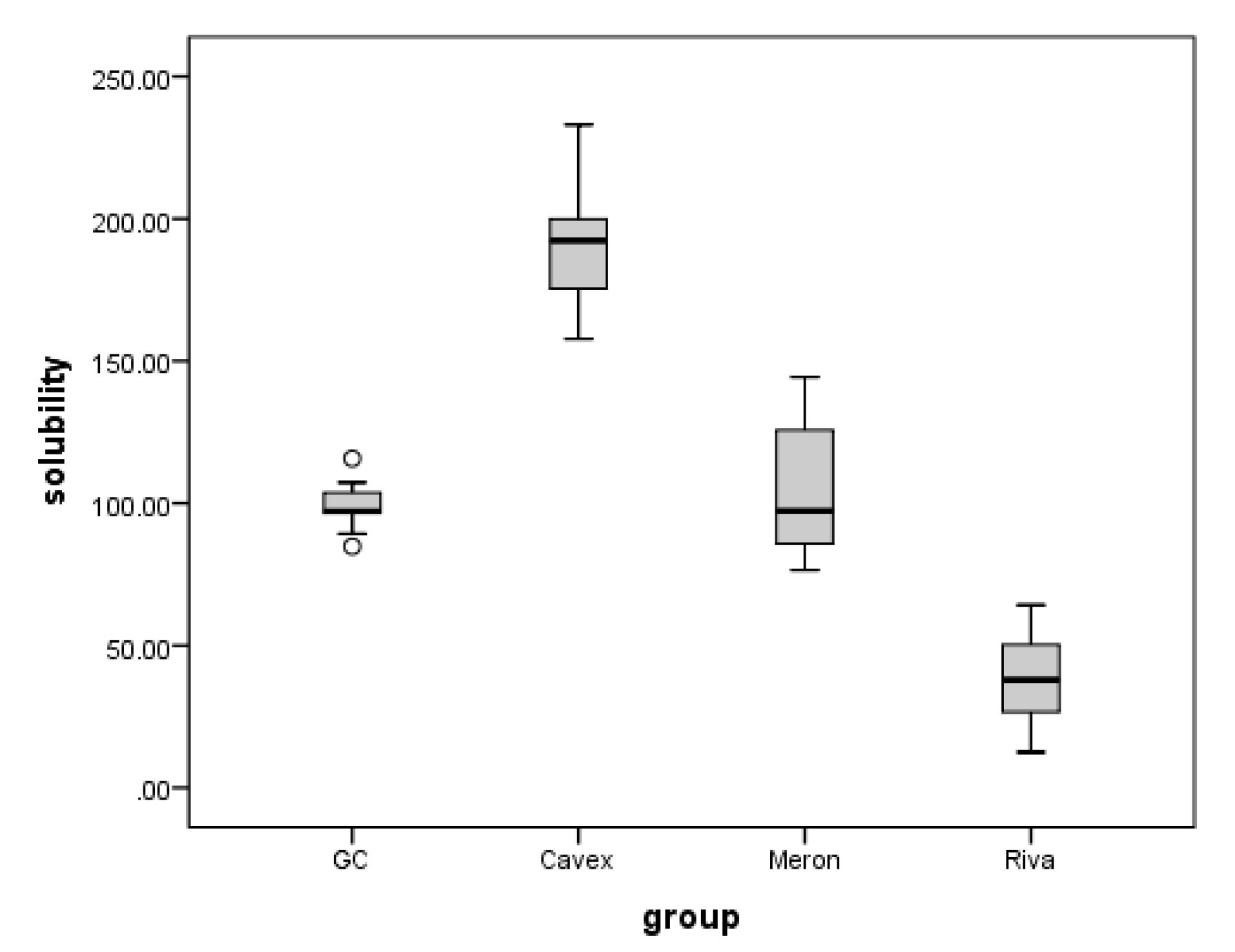
Figure 1.
Box Plot Comparing Solubility in Different Groups.
.
Box Plot Comparing Solubility in Different Groups.
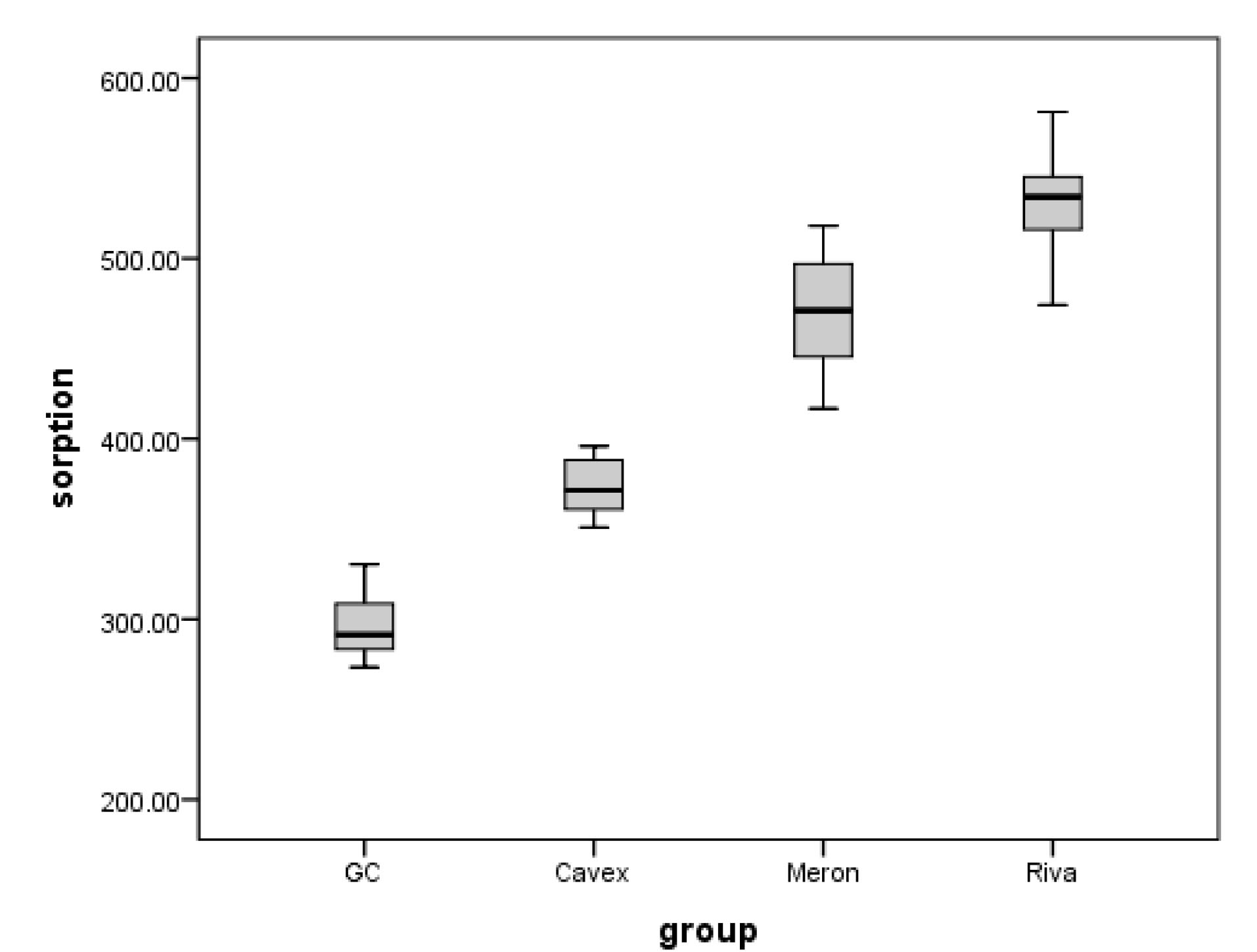
Figure 2.
Box Plot Comparing Sorption in Different Groups.
.
Box Plot Comparing Sorption in Different Groups.
Discussion
Restorations prepared outside the patient’s mouth using the indirect techniques need a luting agent to be cemented in the mouth and to fill cement space between the restoration and the permanent tooth (9). Sorption and solubility are crucial factors that enhance the longevity of the bonded restorations when they are at an optimum level. The probability that dental cements suffer dissolution in the mouth is of a considerable concern to prosthodontists restoring the teeth (6). Zinc phosphate, polyacid-modified resin cement, polycarboxylate cement, and resin-modified glass ionomer cements have been already studied, but the physicochemical characteristics of glass ionomer cements have not received sufficient research attention so far (10). In this study, therefore, water sorption and solubility of four conventional glass ionomer luting were examined and compared. Overall, our study results revealed that the Riva Luting followed by GC Fuji 1 presented statistically higher water sorption values than other cements. As for solubility, Cavex followed by GC Fuji1 showed the highest values. In addition, Meron had the lowest water sorption among all glass ionomer cements.and Riva luting followed by Meron had the lower water solubility.
Huang et al (11) have determined that water sorption is a continuous process that causes an increase in the volume of materials over time. Also, Archegas et al have reported greater water sorption when prolonged storage occurs (12). Due to the hydrogel formation of glass-ionomer materials, they strongly absorb a greater amount of water compared to resin-based materials (12). Water sorption of glass ionomer cements can be attributed to two factors: (1) these materials have sodium in their components that forms water-soluble salts with the matrix anions, and (2) the materials include free calcium and aluminum ions that are present and can be removed by chemical reactions (13). GC Fuji 1 and Riva Luting cements showed the highest water sorption. These results implied that Fuji Ionomer I and Riva Luting cement were more sensitive to water contact. Comparing the powder liquid ratios of the luting cements used in this study revealed that Fuji Ionomer I cement and Riva Luting had the lowest powder/liquid ratios (14). Previous studies have already confirmed that calcium and aluminum ions are rapidly bound into the cement matrix, and some polyacrylate anions are lost in the early stages of cement formation. The ions elusion increased when the powder/liquid ratio decreased. Thus, more sorption for Fuji Ionomer I and Riva Luting cement could be attributed to their lower powder/liquid ratios (14).
Due to the hydrogel formation of the glass-ionomer, they strongly absorb a greater amount of water compared to resin-based materials. The glass-ionomer luting cement, Meron, presented the lowest sorption among all glass ionomer cements. Although Meron C – compared to other types of cements – contains water in its composition, it has the lowest sorption and relative solubility among glass ionomer luting cements (15). It is difficult to map the data for sorption and solubility from one study onto those from other studies and, therefore, varying research results have been generated on these two properties due to the different time periods spent and the different units of measurement adopted.
Cavex and CG Fuji1 cements showed the highest solubility among glass ionomer cements, and cavex showed higher solubility than the other glass ionomer cements, GC Fuji 1. This may have been due to the application of a different filer in Cavex cement. Our study results were also in line with those from Ghasemi et al showing that increasing the storage time decreased the stability of Cavex impression material (16). Previous studies have suggested that GC Fuji 1 requires 3-4 weeks for stabilization in water and has the highest water sorption and solubility compared to other glass ionomer and resin-modified cements (6). The solubility of cement components has a potential impact on both its structural stability and biocompatibility. The rate of dissolution can be influenced by the conditions of the test; therefore, a standardized method similar to the oral cavity condition was adopted in the present study (6). Glass ionomer cements are hydrophilic and high water sorption of this type of material has the least amount of solubility. Our study result indicated an improved solubility of Riva Luting glass ionomer, which was in agreement with the finding from a study by Knobloch et al. Negative values of solubility of glass-ionomer cements demonstrated the high capability of water absorption and masking solubility (17).
Conclusions
Significant differences were detected among all the materials regarding the weight of glass ionomer cements. Furthermore, our study demonstrated that Riva Luting followed by GC Fuji 1 had the highest water sorption, and the solubility was significantly higher in Cavex followed by GC Fuji1. Meron had the lowest water sorption among all glass ionomer cements.and Riva luting followed by Meron had the lower water solubility.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Ethical Statement
Not applicable.
Authors’ Contribution
Study concept and design: AS.
acquisition of data; results interpretation, and manuscript preparation: FA
statistical analysis: MF.
study supervision:AS, AI, SA, AS
References
- Fareed MA, Stamboulis A. Nanoclay addition to a conventional glass ionomer cements: influence on physical properties. Eur J Dent 2014; 8(4):456-63. doi: 10.4103/1305-7456.143619 [Crossref] [ Google Scholar]
- Stamboulis A, Hill RG, Law RV. Structural characterization of fluorine containing glasses by 19F, 27Al, 29Si and 31P MAS–NMR spectroscopy. J Non Cryst Solids 2005; 351(40-42):3289-95. doi: 10.1016/j.jnoncrysol.2005.07.029 [Crossref] [ Google Scholar]
- Carvalho-Júnior JR, Guimarães LF, Correr-Sobrinho L, Pécora JD, Sousa-Neto MD. Evaluation of solubility, disintegration, and dimensional alterations of a glass ionomer root canal sealer. Braz Dent J 2003; 14(2):114-8. doi: 10.1590/s0103-64402003000200008 [Crossref] [ Google Scholar]
- Reis AF, Giannini M, Pereira PN. Influence of water-storage time on the sorption and solubility behavior of current adhesives and primer/adhesive mixtures. Oper Dent 2007; 32(1):53-9. doi: 10.2341/06-13 [Crossref] [ Google Scholar]
- Mortier E, Gerdolle DA, Jacquot B, Panighi MM. Importance of water sorption and solubility studies for couple bonding agent--resin-based filling material. Oper Dent 2004; 29(6):669-76. [ Google Scholar]
- Meşe A, Burrow MF, Tyas MJ. Sorption and solubility of luting cements in different solutions. Dent Mater J 2008; 27(5):702-9. doi: 10.4012/dmj.27.702 [Crossref] [ Google Scholar]
- Giti R, Vojdani M, Abduo J, Bagheri R. The comparison of sorption and solubility behavior of four different resin luting cements in different storage media. J Dent (Shiraz) 2016; 17(2):91-7. [ Google Scholar]
- Nagaraja Upadhya P, Kishore G. Glass ionomer cement: the different generations. Trends Biomater Artif Organs 2005; 18(2):158-65. [ Google Scholar]
- Gemalmaz D, Yoruc B, Ozcan M, Alkumru HN. Effect of early water contact on solubility of glass ionomer luting cements. J Prosthet Dent 1998; 80(4):474-8. doi: 10.1016/s0022-3913(98)70014-9 [Crossref] [ Google Scholar]
- Kvam E, Broch J, Nissen-Meyer IH. Comparison between a zinc phosphate cement and a glass ionomer cement for cementation of orthodontic bands. Eur J Orthod 1983; 5(4):307-13. doi: 10.1093/ejo/5.4.307 [Crossref] [ Google Scholar]
- Huang C, Kei LH, Wei SH, Cheung GS, Tay FR, Pashley DH. The influence of hygroscopic expansion of resin-based restorative materials on artificial gap reduction. J Adhes Dent 2002; 4(1):61-71. [ Google Scholar]
- Archegas LR, Caldas DB, Rached RN, Vieira S, Souza EM. Sorption and solubility of composites cured with quartz-tungsten halogen and light emitting diode light-curing units. J Contemp Dent Pract 2008; 9(2):73-80. [ Google Scholar]
- Crisp S, Lewis BG, Wilson AD. Characterization of glass--ionomer cements 6 A study of erosion and water absorption in both neutral and acidic media. J Dent 1980; 8(1):68-74. doi: 10.1016/s0300-5712(80)80046-7 [Crossref] [ Google Scholar]
- Crisp S, Lewis BG, Wilson AD. Glass ionomer cements: chemistry of erosion. J Dent Res 1976; 55(6):1032-41. doi: 10.1177/00220345760550060501 [Crossref] [ Google Scholar]
- Gerdolle DA, Mortier E, Jacquot B, Panighi MM. Water sorption and water solubility of current luting cements: an in vitro study. Quintessence Int 2008; 39(3):e107-14. [ Google Scholar]
- Ghasemi E, Ebadian B, Badrian H, Asadi A. Effect of storage time on dimensional changes of irreversible hydrocolloid impression material. Journal of Isfahan Dental School 2012; 7(5):585-91. [ Google Scholar]
- Knobloch LA, Kerby RE, McMillen K, Clelland N. Solubility and sorption of resin-based luting cements. Oper Dent 2000; 25(5):434-40. [ Google Scholar]