Avicenna J Dent Res. 13(4):124-129.
doi: 10.34172/ajdr.2021.24
Original Article
Evaluation of the Relation of Smoking, Gallstones, and Renal Stones With Sialolithiasis in Patients Referred to Oral Medicine and ENT Department of Tabriz University of Medical Sciences
Farzaneh Pakdel 1, Rana Attaran 2, Sevda Movafagh 3, Zahra Aghazadeh 4, * 
Author information:
1Department of Oral Medicine, Faculty of Dentistry, University of Tabriz Medical Sciences, Tabriz, Iran.
2Oral Medicine Specialist, Tabriz, Iran.
3Under Graduate Student, Faculty of Dentistry, University of Tabriz Medical Sciences, Tabriz, Iran.
4Stem Cell Research Center, University of Tabriz Medical Sciences, Tabriz, Iran.
Abstract
Background: The exact mechanism of the formation of salivary gland stones is unknown. Elucidating pathophysiology of the formation of salivary stones might prevent both their formation and the need for implementing invasive surgical procedures. Therefore, this study aimed to evaluate the effects exerted by some etiological factors on the formation of salivary gland stones.
Methods: In this case–control study, the records of 80 patients with sialolithiasis were studied as a census from April 2011 to June 2019. These patients were referred to the Oral Medicine and the ENT departments of Tabriz University of Medical Sciences. The control group consisted of the same number of the patients with no sialolithiasis. Two groups were compared in terms of stone size, smoking, gallstones, and renal stones. Chi-squared, independent t-test, and Mann-Whitney U test were adopted to examine the quantitative variables. The data were analyzed using SPSS 17. Statistical significance was set at P<0.05.
Results: Overall, 96.2% of sialoliths were found in the submandibular gland, of which 78.8% were single. Moreover, 32.5% of the patients with a history of sialolithiasis were smokers, whereas this frequency was 23.8% in the control group. In the case and control groups, 2.5% and 5% of the patients had a history of renal stones, respectively. Only one patient who had undergone a surgical procedure to remove salivary gland stones had a history of gallstones, while none of the patients in the control group had a history of gallstones.
Conclusions: The results showed that the formation of salivary gland stones was not associated with smoking, history of renal stones, and gallstones. Furthermore, it was found that the numbers and sizes of salivary stones were not affected by smoking.
Keywords: Gallstones, Renal stones, Sialolithiasis, Smoking
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Pakdel F, Attaran R, Movafagh S, Aghazadeh Z. Evaluation of the relation of smoking, gallstones, and renal stones with sialolithiasis in patients referred to oral medicine and ENT department of tabriz university of medical sciences. Avicenna J Dent Res. 2021;13(4):124-129. doi: 10.34172/ajdr.2021.24.
Background
Highlights
-
The formation of salivary gland stones seemed to not be in association with smoking, history of renal stones, and gallstones.
-
The numbers and sizes of salivary stones were not affected by smoking.
Salivary gland stones or sialoliths are single or multiple calcified structures found in the parenchyma or ducts of the salivary gland (1,2). The salivary stones are formed through the partial or complete obstruction of salivary ducts, leading to recurrent salivary gland swelling, acute or chronic bacterial infections, or mucocele formation. Sialoliths are often detected in the submandibular gland duct (75%–92%), in the parotid gland duct (28%–48%), and rarely in the sublingual glands or minor salivary gland ducts (3). This problem is more prevalent in the fourth and fifth decades of life and rarely occurs among children. Approximately 1% of sialolithiasis cases is familial. According to a recent study, salivary gland stones within males and females show a similar distribution (4). As for the patients with salivary gland stones which cannot be removed or destroyed within the gland, it is necessary to remove the salivary gland.
The exact mechanism of the formation of salivary gland stones is still unknown (5). Understanding the pathophysiology of sialolith formation might prove useful in preventing their occurrence and eliminating the need for invasive surgical procedures. Although the etiology and pathogenesis of sialoliths are unknown, several theories have been derived from the analysis of removed stones. Salivary stasis or even decreased salivary flow has been suggested as a factor responsible for the formation of salivary stones. Moreover, inflammation or sialadenitis has been implicated in the formation of salivary stones. Factors such as tobacco use, decreased fluid intake, and drugs that reduce saliva flow appear to be effective in the inflammation of salivary glands (5).
Furthermore, some researchers have suggested that sialoliths and renal stones might co-occur due to the similarities between their predisposing factors (1,6). It is also possible that the pathogenesis of gallstones and sialoliths might exhibit some similarities (3,7). Lustmann et al have reported that the prevalence of renal stones in 56 patients with sialolithiasis was 10.7 %, which is higher than that in a healthy population, indicating a relationship between renal and salivary gland stones (8). Some studies have shown a significant relationship between salivary gland stones and renal stones, indicating that the underlying factors of these two diseases are common (3,6). However, other studies have failed to report a higher prevalence of renal stones and gallstones in patients with salivary gland stones (1). In a study performed at the University of California, for instance, no higher prevalence of gallstones and renal stones was found in the healthy population after studying the medical history of 153 patients with salivary gland stones. In the given study, it was claimed that the risk factors and pathogenesis of these conditions were more likely different from each other (2). In another study, no relationship was detected between renal stones, gallstones, diabetes mellitus, and high blood pressure and sialolithiasis (1).
Some studies have reported a higher rate of smoking in patients with sialolithiasis (1,2). Tobacco use leads to inflammation and subsequent formation of mucosal plaque and calcified masses between the ducts, leading to the formation of salivary gland stones (1,5).
Considering the statistical discrepancies in previous studies, this study aimed to investigate the possible relationship between salivary gland stones, renal stones, and gallstones and smoking in patients with sialolithiasis, and to compare them with a healthy group.
Materials and Methods
The medical records of these centers and all the patient files with sialolithiasis were studied as a census. The record evaluation was carried out by oral medicine specialists. All the files were selected from among the files on patients referring to the Department of Oral Medicine or ENT Department of Imam Reza hospital from April 2011 to June 2019. As for the control group, the files on patients with no sialolithiasis were examined in the same departments. The subjects in the two groups were matched in terms of age and sex and according to the inclusion and exclusion criteria. The two groups were evaluated and compared in terms of stone size, smoking, gallstones, and renal stones.
Inclusion Criteria
1. Adult patients >18 years of age, both males and females with salivary gland stones in the case group and without salivary gland stones in the control group.
Exclusion Criteria
-
Subjects <18 years of age
-
Thyroid and diuretic drug users
-
Patients with hyperparathyroidism
-
Patients with other systemic problems such as diabetes, autoimmune disease, and hypertension.
In this study, only the records containing the patient’s informed written consent for using his or her medical information in medical research were investigated.
All the adult patients (>18 years of age) entered the study as cases, and the patients’ demographic data, including sex, age, and additional information about the number, size, and location of sialoliths, were recorded on the checklist for the study group. In the control group, the files of patients with no sialolithiasis were examined. These controls matched with the cases in terms of age and sex. The demographic data of this group were also registered on the checklist.
Data Analysis
The results were reported using descriptive statistics and means ± standard deviations for quantitative variables, as well as frequencies for qualitative variables. Chi-squared test was used to compare smoking and the presence of renal stones and gallstones in the two groups with and without sialolithiasis. Independent t test and Mann-Whitney U test were applied to evaluate the quantitative variables. The results were analyzed using SPSS 17. Statistical significance was set at P<0.05.
Results
Eighty patients were evaluated in each group, of which 53 were males (66.2%) and 27 (33.7%) were females. The mean age of the subjects was 41 years in the case group, and 42 years in the control group (Table 1). Due to the non-normal distribution of age in the two groups, independent t test was used to investigate the mean age difference, which was not significant between the case and control groups (P = 0.484).
Table 1.
The Mean Age of the Male and Female Subjects
|
|
|
Minimum Age
|
Maximum Age
|
Mean ± SD of Age
|
P
Value
|
| Case |
Female |
20 |
64 |
41.44±13.36 |
41.17±2.49 |
0.484 |
| Male |
21 |
78 |
41.03±12.15 |
| Control |
Female |
24 |
70 |
41.85±13.55 |
42.61±13.41 |
| Male |
18 |
76 |
43.00±13.45 |
Out of 80 patients in the case group, 77 patients (96.2%) had salivary gland stones in the submandibular gland, two patients (2.5%) in the parotid gland, and one patient (1.2%) in the sublingual gland (Table 2, Figure 1).
Table 2.
The Distribution of Salivary Gland Stones Among the Different Salivary Glands
|
Location of Sialolith
|
Parotid Gland
|
Sublingual Gland
|
Submandibular Gland
|
| Number (%) of identified sialoliths |
2 (2.5%) |
1 (1.2%) |
77 (96.2%) |
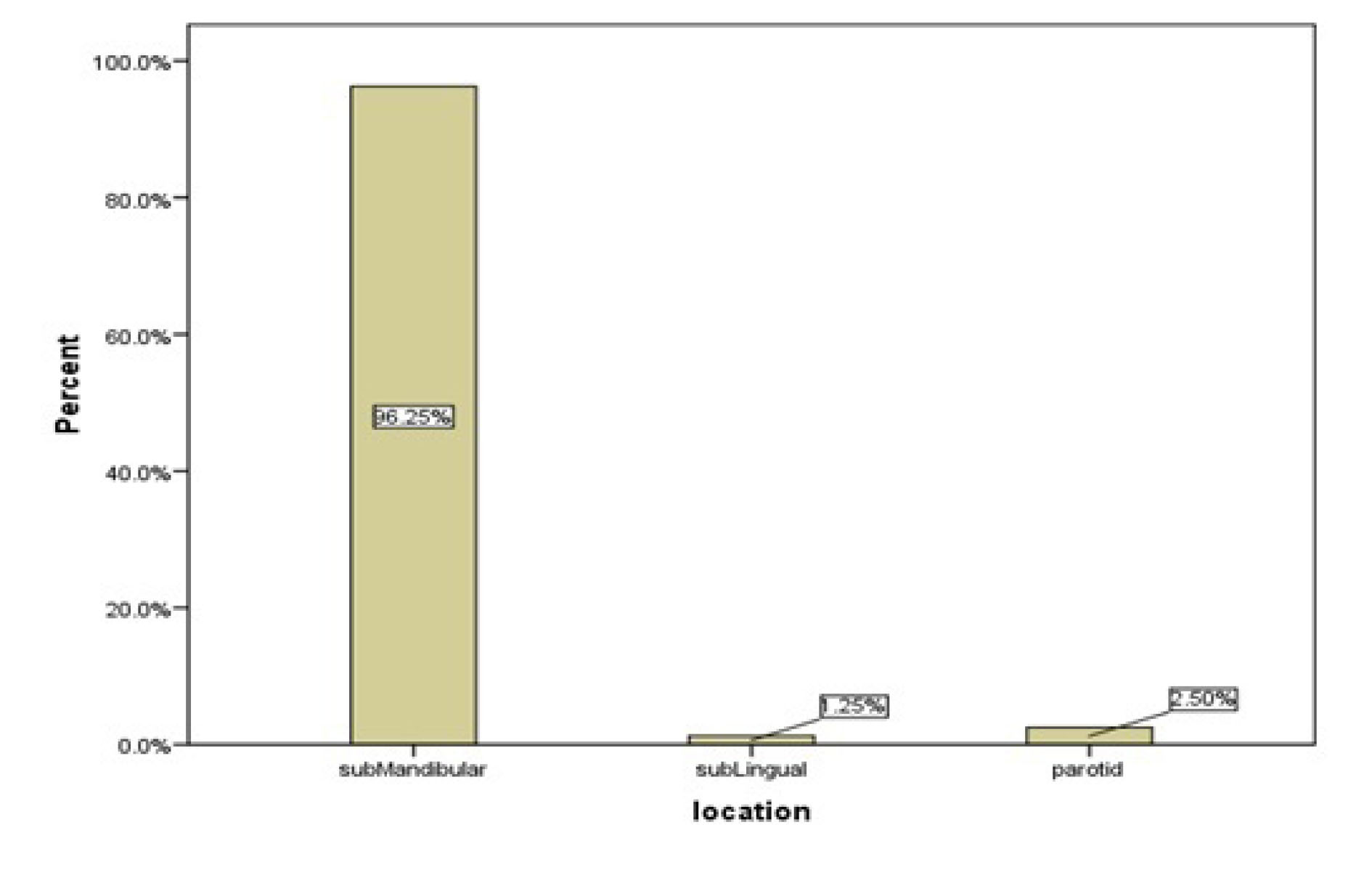
Figure 1.
The Distribution of Salivary Gland Stones Among the Different Salivary Glands.
.
The Distribution of Salivary Gland Stones Among the Different Salivary Glands.
As for the 80 sialolithiasis cases, 63 patients (78.8%) were found to have only one stone, and 10 patients (12.5%) were detected to carry several sialoliths. In 7 cases (8.8%), the number of sialoliths had not been recorded (Table 3).
Table 3.
The Frequencies of Salivary Gland Stones
|
Multiple
|
Single
|
Not Recorded
|
| 63 (78.8%) |
10 (12.5%) |
7 (8>9%) |
In the case group, 26 (32.5%) patients were smokers, while in the control group, 19 patients (23.8%) had a smoking habit (Table 4). Although the number of cigarettes per day was not mentioned in the records, the “heavy smoker” section was not filled for any of the patients. In other words, of all the identified smokers, 57.78% were in the case (sialolithiasis) group, and 42.22% were in the control (non-sialolithiasis) group (Figure 2). According to the chi-squared test, no significant effect of smoking on the formation of salivary stones was observed (P= 0.218).
Table 4.
Frequencies of Smokers in the Study Population
|
The Number of Smokers
|
|
P
Value
|
| Case |
26 (32.5%) |
0.218 |
| Control |
19 (23.8%) |
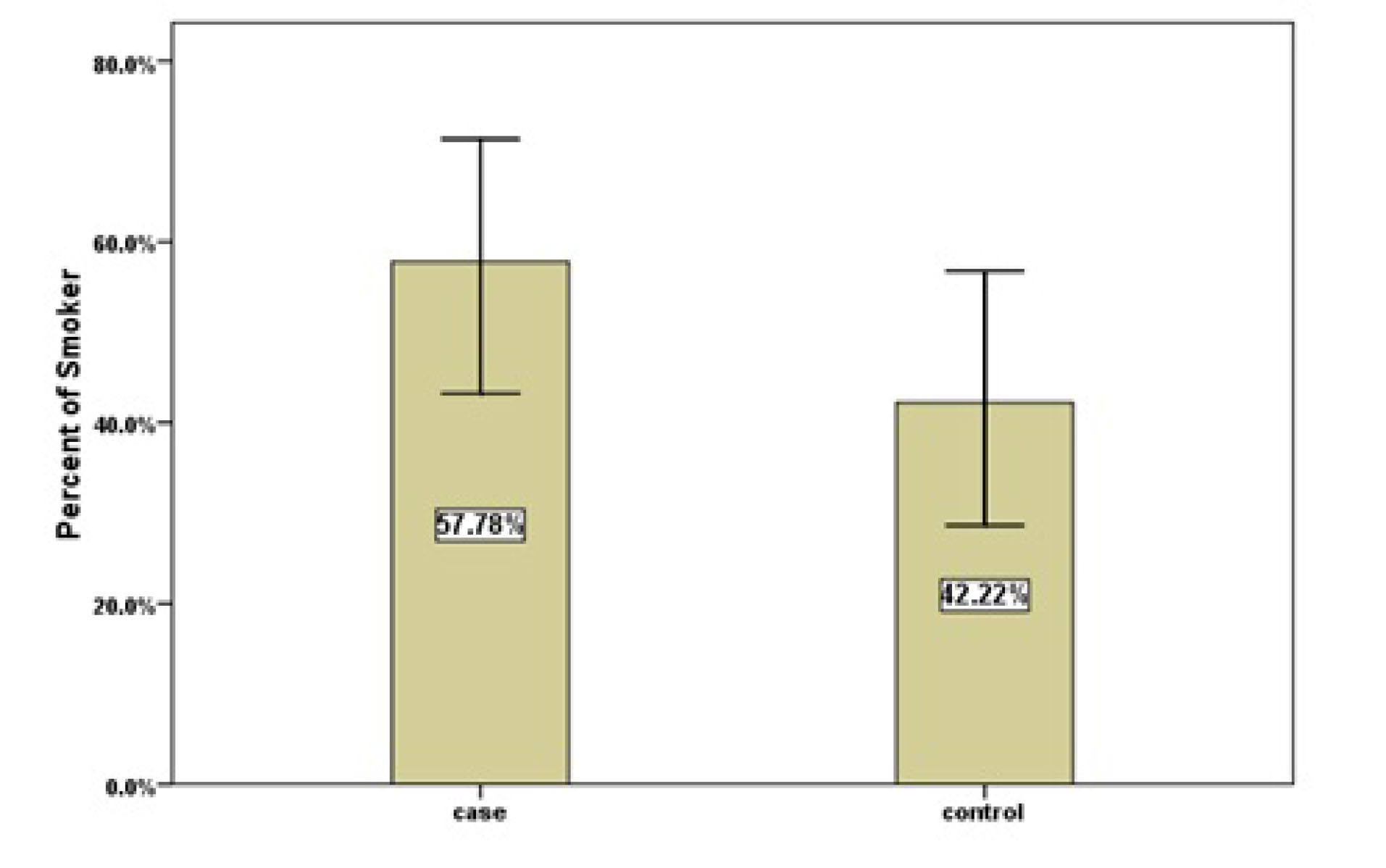
Figure 2.
Frequencies of Smokers in the Case and Control Groups.
.
Frequencies of Smokers in the Case and Control Groups.
Out of all the patients with sialolithiasis, two patients (2.5%) had a history of renal stones, whereas, in the control group, four patients (5%) had a history of renal stones (Table 5). Chi-squared test showed no significant difference between the case and control groups in terms of renal stones (P= 0.681).
Table 5.
Frequencies of the History of Renal Stones and Gallstones
|
|
Case
|
Control
|
P
Value
|
| A history of renal stones |
2 (2.5%) |
4 (5.0%) |
0.681 |
| A history of gallstones |
1 (1.2%) |
0 (0.0%) |
0.99 |
Only one patient (1.2%) had a history of gallstones, and none of the subjects in control group mentioned a history of gallstones. Chi-squared test showed no significant difference between the case and control groups gallstones (P= 0.99).
In the current study, the largest and smallest stones removed by surgery were 20 mm and 3 mm in diameter, respectively. The mean size of the surgically removed stones was 9.8 mm.
The mean diameters of salivary gland stones removed in smokers and non-smokers were 11.25 and 8.20 mm, respectively (Table 6). Due to the non-normal distribution of sizes in the groups (P<0.05), Mann-Whitney U test was used to investigate the difference in the size of sialoliths between smokers and non-smokers, which showed no significant difference between these values (P= 0.383).
Table 6.
Mean Sizes of Salivary Gland Stones in Smokers and Non-smokers
|
Sialolith Size
|
Mean ± SD
|
Minimum Size
|
Maximum Size
|
P
Value
|
| Smokers |
11.25 ± 6.15 |
4 mm |
20 mm |
0.383 |
| Non-smokers |
8.20 ± 4.37 |
3 mm |
16 mm |
Out of 10 patients with multiple salivary gland stones, four were smokers, and six were non-smokers (Table 7). Chi-squared test was used to investigate the difference in the number of salivary gland stones between smokers and non-smokers. The results revealed no significant difference between the two groups (P= 0.851).
Table 7.
Frequencies of Sialoliths in Smokers and Non-smokers Patients
|
|
One Sialolith
|
>1 Sialolith
|
Not Recorded
|
P
Value
|
| Smokers |
20 (31.7%) |
4 (40%) |
2 (28.6%) |
0.851 |
| Non-smokers |
43 (68.3%) |
6 (60%) |
5 (71.4%) |
Discussion
Sialolithiasis is a common disorder of the salivary glands, and accounts for nearly one-third of salivary gland disorders (4). The exact mechanism for the formation of salivary gland stones still requires sufficient elucidation (3). Therefore, this study aimed to compare the etiological factors responsible for the formation of salivary gland stones in patients with or without sialolithiasis. Salivary gland stones often affect people over the age of 40, and are rarely observed in children (1); in the present study, accordingly, the mean age of the subjects in the case group was 41.
A few studies have already shown an almost equal distribution between males and females (4). However, studies conducted before 2000, or a study by Kraaij et al found that the prevalence of sialolithiasis in males was higher than that in females (1). In our study, 80 patients were evaluated in each group, of whom 53 were male (66.2%) and 27 (33.7%) were female, and the prevalence in males was detected to be almost twice higher than that in females.
Salivary gland stones are more common in the submandibular duct system (72%–95%) compared to that in the parotid gland (4%–28%), and are rarely observed in the sublingual and minor salivary glands (1). In the case group of the present study, 77 (96.2%) patients had salivary gland stones in the submandibular gland; two (2.5%) patients had them in the parotid gland, with one (1.2%) in the sublingual gland, which were consistent with the available data from other studies in this subject area. The submandibular gland is the most common salivary gland to be affected by sialoliths due to its long and thick duct (2,5,7,9). In addition, the narrow orifice of Wharton’s duct and its path, which is against gravity, have also been reported as the causes of this distribution (10,11).
The current study showed that 32.5% of the patients in the case group, who had undergone surgery to remove the stone, were smokers; however, 23.8% of the patients in the control group were smokers. Chi-square test showed no significant difference between the case and the control groups in terms of smoking. Therefore, our study demonstrated that smoking had not increased the risk of developing sialolithiasis, which was consistent with the results reported by Yiu et al (%). However, Kraaij et al revealed that smoking was more prevalent in patients with sialolithiasis and concluded that smoking increased the risk of sialolithiasis (1). Huoh et al indicated that smoking was more common among patients with sialolithiasis than healthy individuals; however, the difference was not significant. In this study, nonetheless, smoking and diuretic use could play an important role in the formation of salivary gland stones (2). Since the sample sizes in the two studies were 208 and 153, respectively, it was possible to achieve similar results with an increase in the sample size.
Yiu et al evaluated the relationship between the number of stones and the use of cigarettes. The results revealed no significant difference in the number of salivary gland stones between smokers and non-smokers (5). In the current study, chi-squared test was used to investigate the difference in salivary gland stone numbers between smokers and non-smokers, which indicated that smoking had not affected the number of sialoliths, and the number of salivary gland stones had not exhibited any association with smoking.
Huoh et al reported that submandibular stones in smokers (with a mean diameter of 8.7 mm) were slightly larger than that in non-smokers (with a mean diameter of 7.9 mm) (2). Yiu et al showed that smokers (12.4 mm) had larger stones than those having kicked the smoking habit (7.5 mm), which could be attributed to two factors: the first factor decreased salivary flow; and the second one, smoking could have increased bacterial load in the salivary gland duct or the gland itself by reducing antibacterial activity (5).
In the current study, the evaluation of sialolith size in smokers and non-smokers showed no significant difference between the two groups. Our study result was different from that reported by above-mentioned studies, which might be attributed to an inadequate number of patient files evaluated.
Zenk et al examined 635 patients with sialolithiasis and reported no higher prevalence of renal stones and gallstones in these patients (12). Moreover, in a retrospective cohort study on the medical history of 153 patients with sialolithiasis, the prevalence of gallstones and renal stones in patients with sialolithiasis was not significantly higher than that in healthy individuals (2). Kraaij et al investigated 208 patients with sialolithiasis and reported that the prevalence of renal stones and gallstones in the case group was not significantly different from that in the control group (1). However, Wu et al carried out a case–control study and determined that sialolithiasis had a significant association with a history of renal stones (6). Also, Grases et al studied the structure and composition of submandibular gland stones and indicated that the microstructures and macrostructures of hydroxylapatite in salivary calculi were more or less similar to those of renal calculi. They showed similarities between the two calcific centers and assumed that the salivary gland and renal stones were likely to have the same mechanism of development (13). In earlier studies, Lustmann et al revealed that the prevalence of renal stones in patients with sialolithiasis was higher than that in the healthy population, possibly indicating an association between the two conditions (8). The case-control study by Hung et al demonstrated that patients with a history of gallstones were more likely to develop sialolithiasis. The authors offered no scientific evidence to support their findings.
Wu et al and Hung et al reported that the target populations in different geographic areas could impact the results; therefore, the data could not be generalized to other tribes and populations (6). Taking into consideration the findings from previous studies (1,6,8,13), it seemed that studies with large sample sizes had achieved better results in different fields.
The current study showed that salivary gland stones were more prevalent in the submandibular, parotid, and sublingual glands, respectively, in descending order. It was observed that stones were mostly single and, in a few cases, multiple. The number of patients who smoked and had salivary gland stones and underwent surgery to remove the stones was higher than non-smokers. No significant relationships were discovered among salivary gland stones, gallstones, and renal stones. The salivary gland stones were larger in smokers compared to non-smokers. However, the difference was not significant (P = 0.383). The present study faced few limitations as follows: first, the pattern of smoking habits was not clear in the patient files. Second, identifying the relationship between the number of cigarettes per day and the duration of this habit was not possible in this investigation; however, the identification could absolutely prove beneficial. It is therefore recommended that, besides the pattern of smoking, a study with a large number of patients in different geographical locations be conducted. Moreover, performing a systematic review appears to be helpful considering the controversial results reported by different studies.
Conclusions
According to the results from this study, the formation of salivary gland stones was not associated with smoking, history of renal stones, and gallstones. Furthermore, the numbers and sizes of salivary stones were not affected by smoking. However, finding a clear relationship between these factors and the formation of salivary gland stones requires more investigations.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Ethical Statement
The protocol of this cross-sectional and case-control study was approved by the Ethics Committee of Tabriz University of Medical Sciences under the code IR.TBZMED.REC.1396.119. This study complied with the ethical standard recommended by the Helsinki declaration.
Authors’ Contribution
FP and RA conceived of the presented idea. Both of them developed the theory and performed the computations. SM was responsible for completing the check lists. ZA and RA verified the analytic methods. FP supervised the findings of this work. All authors discussed the results and contributed to the final manuscript. ZA was responsible for writing the article and performing the publication process.
References
- Kraaij S, Karagozoglu KH, Kenter YA, Pijpe J, Gilijamse M, Brand HS. Systemic diseases and the risk of developing salivary stones: a case control study. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 119(5):539-43. doi: 10.1016/j.oooo.2015.01.010 [Crossref] [ Google Scholar]
- Huoh KC, Eisele DW. Etiologic factors in sialolithiasis. Otolaryngol Head Neck Surg 2011; 145(6):935-9. doi: 10.1177/0194599811415489 [Crossref] [ Google Scholar]
- Hung SH, Lin HC, Su CH, Chung SD. Association of sialolithiasis with cholelithiasis: a population-based study. Head Neck 2016; 38(4):560-3. doi: 10.1002/hed.23926 [Crossref] [ Google Scholar]
- Kraaij S, Karagozoglu KH, Forouzanfar T, Veerman EC, Brand HS. Salivary stones: symptoms, aetiology, biochemical composition and treatment. Br Dent J 2014; 217(11):E23. doi: 10.1038/sj.bdj.2014.1054 [Crossref] [ Google Scholar]
- Yiu AJ, Kalejaiye A, Amdur RL, Todd Hesham HN, Bandyopadhyay BC. Association of serum electrolytes and smoking with salivary gland stone formation. Int J Oral Maxillofac Surg 2016; 45(6):764-8. doi: 10.1016/j.ijom.2016.02.007 [Crossref] [ Google Scholar]
- Wu CC, Hung SH, Lin HC, Lee CZ, Lee HC, Chung SD. Sialolithiasis is associated with nephrolithiasis: a case-control study. Acta Otolaryngol 2016; 136(5):497-500. doi: 10.3109/00016489.2015.1129068 [Crossref] [ Google Scholar]
- Kim SY, Kim HJ, Lim H, Lim MS, Kim M, Park IS. Association between cholelithiasis and sialolithiasis: two longitudinal follow-up studies. Medicine (Baltimore) 2019; 98(25):e16153. doi: 10.1097/md.0000000000016153 [Crossref] [ Google Scholar]
- Lustmann J, Regev E, Melamed Y. Sialolithiasis A survey on 245 patients and a review of the literature. Int J Oral Maxillofac Surg 1990; 19(3):135-8. doi: 10.1016/s0901-5027(05)80127-4 [Crossref] [ Google Scholar]
- Madani G, Beale T. Inflammatory conditions of the salivary glands. Semin Ultrasound CT MR 2006; 27(6):440-51. doi: 10.1053/j.sult.2006.09.005 [Crossref] [ Google Scholar]
- Baurmash HD. Submandibular salivary stones: current management modalities. J Oral Maxillofac Surg 2004; 62(3):369-78. doi: 10.1016/j.joms.2003.05.011 [Crossref] [ Google Scholar]
- Mimura M, Tanaka N, Ichinose S, Kimijima Y, Amagasa T. Possible etiology of calculi formation in salivary glands: biophysical analysis of calculus. Med Mol Morphol 2005; 38(3):189-95. doi: 10.1007/s00795-005-0290-7 [Crossref] [ Google Scholar]
- Zenk J, Constantinidis J, Kydles S, Hornung J, Iro H. [Clinical and diagnostic findings of sialolithiasis]. HNO 1999; 47(11):963-9. doi: 10.1007/s001060050476.[German] [Crossref] [ Google Scholar]
- Grases F, Santiago C, Simonet BM, Costa-Bauzá A. Sialolithiasis: mechanism of calculi formation and etiologic factors. Clin Chim Acta 2003; 334(1-2):131-6. doi: 10.1016/s0009-8981(03)00227-4 [Crossref] [ Google Scholar]