Avicenna J Dent Res. 16(3):161-169.
doi: 10.34172/ajdr.1858
Original Article
Evaluation of the Remineralization Potential of an Herbal Non-fluoridated Dentifrice on Carious Lesions: An In Vitro Study
Paras Mull Gehlot 1, *  , Raghvendra Shanbhog 2
, Raghvendra Shanbhog 2  , Nandlal Bhojraj 3
, Nandlal Bhojraj 3  , Jyoti Singh 4, Mahesh Katariya 4, Prasun Bandyopadhyay 4
, Jyoti Singh 4, Mahesh Katariya 4, Prasun Bandyopadhyay 4
Author information:
1Department of Conservative Dentistry and Endodontics, Member SIG Cariology, JSS Dental College and Hospital, Mysuru
2Department of Pediatric and Preventive Dentistry, Member SIG Cariology, JSS Dental College and Hospital, Mysuru
3Department of Pediatric and Preventive Dentistry, Head, SIG Cariology, JSS Dental College and Hospital, Mysuru
4Dabur Research & Development Centre, Ghaziabad, Uttar Pradesh, India
Abstract
Background: This in vitro study compared the remineralization potential of Dabur red (DR), a non-fluoridated herbal toothpaste, with Colgate Strong Teeth (CST), a fluoride-based toothpaste, on permanent teeth with artificially created white spot lesions. Vickers microhardness (VMH), quantitative light-induced fluorescence system (QLF), and scanning electron microscopy (SEM) were used for data analysis.
Methods: Enamel samples were prepared from the buccal surface of forty-five extracted maxillary premolars and subjected to demineralization to achieve adequate fluorescence loss (ΔF). The samples were divided into DR, CST, and control groups (n=15) and underwent 21 days of pH cycling. VMH was measured at baseline and after 21 days. QLF imaging was performed on days 7, 14, and 21. One randomly selected sample from each group underwent SEM examination to compare morphological variations. The statistical analysis was conducted using SPSS 22.0. Intra-group comparisons were made with repeated measures of analysis of variance (ANOVA), and one-way ANOVA with Tukey’s post-hoc test was utilized for inter-group comparisons (P<0.05).
Results: Mean VHN increased significantly in all three groups from baseline (DR=40.47, CST=41.91, control=36.42) to the 21st day (DR=119.47, CST=120.36, control=74.36) (P=0.00). Significant differences were found between the control and test groups, but not between DR and CST. No statistically significant difference was observed in ΔF at baseline and 7 days, but there were significant differences among the groups at 14 and 21 days. SEM images revealed dense mineralization for both DR and CST.
Conclusion: After 21 days of in vitro remineralization, both DR and Colgate toothpaste demonstrated similar remineralizing potential as evaluated by surface MH and QLF.
Keywords: Remineralization, Herbal toothpaste, Microhardness, Artificial caries, Quantitative light-induced fluorescence
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Gehlot PM, Shanbhog R, Bhojraj N, Singh J, Katariya M, Bandyopadhyay P. Evaluation of the remineralization potential of an herbal non-fluoridated dentifrice on carious lesions: an In vitro study. Avicenna J Dent Res. 2024; 16(3):161-169. doi:10.34172/ajdr.1858
Background
White spot lesions, the earliest stage of dental caries, are caused by the subsurface demineralization of enamel, appearing as opaque white areas due to mineral loss. Effective management is crucial in preventing the progression of cavitation. Remineralizing agents enhance the natural repair process by depositing minerals such as calcium (Ca) and phosphate (P) into the demineralized enamel, restoring mineral content, and improving optical properties to reduce the visibility of lesions. The formation and progression of caries are dynamic disease processes that involve the demineralization and remineralization of enamel and dentin. The demineralization process is initiated when Ca and P ions disintegrate off the tooth surface owing to the secretion of organic acids by acidogenic bacteria in dental plaque at a critical pH (1). The effort to maintain healthy, strong teeth depends on the balance between demineralization and remineralization (1,2).
Saliva is a vital natural barrier against dental caries because it comprises a supersaturated Ca and P solution that neutralizes and dilutes the acid that causes demineralization (2). However, this oral fluid-based natural remineralization process is not strong enough to endure prolonged exposure to intense acidic conditions. Remineralization is demonstrated to aid in these circumstances by an external supply of Ca and P ions, which are 20–30 times more abundant than those found in saliva (3,4). As a result, the remineralization of enamel by the use of several Ca-P-based agents, which are currently offered in a range of commercial formulations, has received extensive attention (5).
The consumer market has shown interest in herbal health goods. Considering that herbal toothpaste is derived from natural sources and has strong antibacterial qualities, some studies recommend using this production instead of synthetic toothpaste (6). The active antimicrobial ingredients and formulations of synthetic toothpastes with the presence of fluoride and other ingredients demonstrate their role in dental care. However, herbal toothpastes have been claimed to significantly reduce oral microbial load after brushing (6). Further, such products are sources of safer and more effective alternatives to chemical toothpaste (7).
The presence of a white-spot lesion is the initial clinical indication of dental caries, and its diagnosis is of prime importance. This is because these lesions can be reversed or arrested, thus reducing the disease’s overall impact (8). Evaluating enamel demineralization and remineralization over time is made possible by several visual and non-destructive techniques (3,4).
Quantitative light-induced fluorescence system (QLF) is one such optical technology that has had extensive validation for its sensitivity and reliability (9,10). QLF works on the principle that enamel will auto-fluoresce in response to light. The loss of fluorescence observed in the demineralized enamel can be detected, quantified, and longitudinally monitored using the QLF.
Over the past few years, there has been a decline in the incidence of tooth demineralization as a result of improved oral hygiene practices and the development of newer toothpastes with improved remineralization potential. Fluoride is believed to be essential for enamel remineralization and cavity prevention, although it initiates the remineralization process only in the presence of sufficient Ca and P (11).
The remineralizing potential of fluoridated toothpastes is limited due to the low concentration of Ca and P in the saliva (12). According to published research, fluoride’s remineralization activity rises in the presence of sufficient free Ca and P ions (13). There is also the potential for fluoride toxicity. A study by Grandjean and Landrigan classified fluoride as a human developmental neurotoxicant, similar to toxic metals such as lead (14). In addition, high fluoride levels should be avoided to reduce the risk of dental fluorosis in children (15).
Despite the abundance of remineralizing agents, exploring new strategies and evaluating various agents are crucial to enhancing the remineralization process. A review of the literature found limited studies comparing the remineralizing potential of non-fluoridated herbal toothpaste with fluoridated toothpaste using microhardness (MH) and QLF analysis.
Hence, the present in vitro investigation aimed to compare the remineralizing potential of two commercially available types of toothpaste, namely, an herbal non-fluoridated toothpaste (Dabur Red, Dabur India Ltd.) and a fluoridated toothpaste (Colgate Strong Teeth, Colgate-Palmolive India Limited). This comparison was conducted in vitro using a surface MH test and the QLF method on artificial carious lesions. The results would help in understanding the caries-reducing effect of herbal, non-fluoridated toothpaste.
Materials and Methods
This in vitro comparative study was performed in the Department of Conservative Dentistry and Endodontics. Figure 1 summarizes the methodological steps involved in the study. A sample size of 15 per group was determined by taking data from related studies that have been published in the literature, using 80% power and a 95% confidence interval (9,12). The study was conducted with 2 interventional groups and 1 control group, each containing 15 samples (12,16,17).
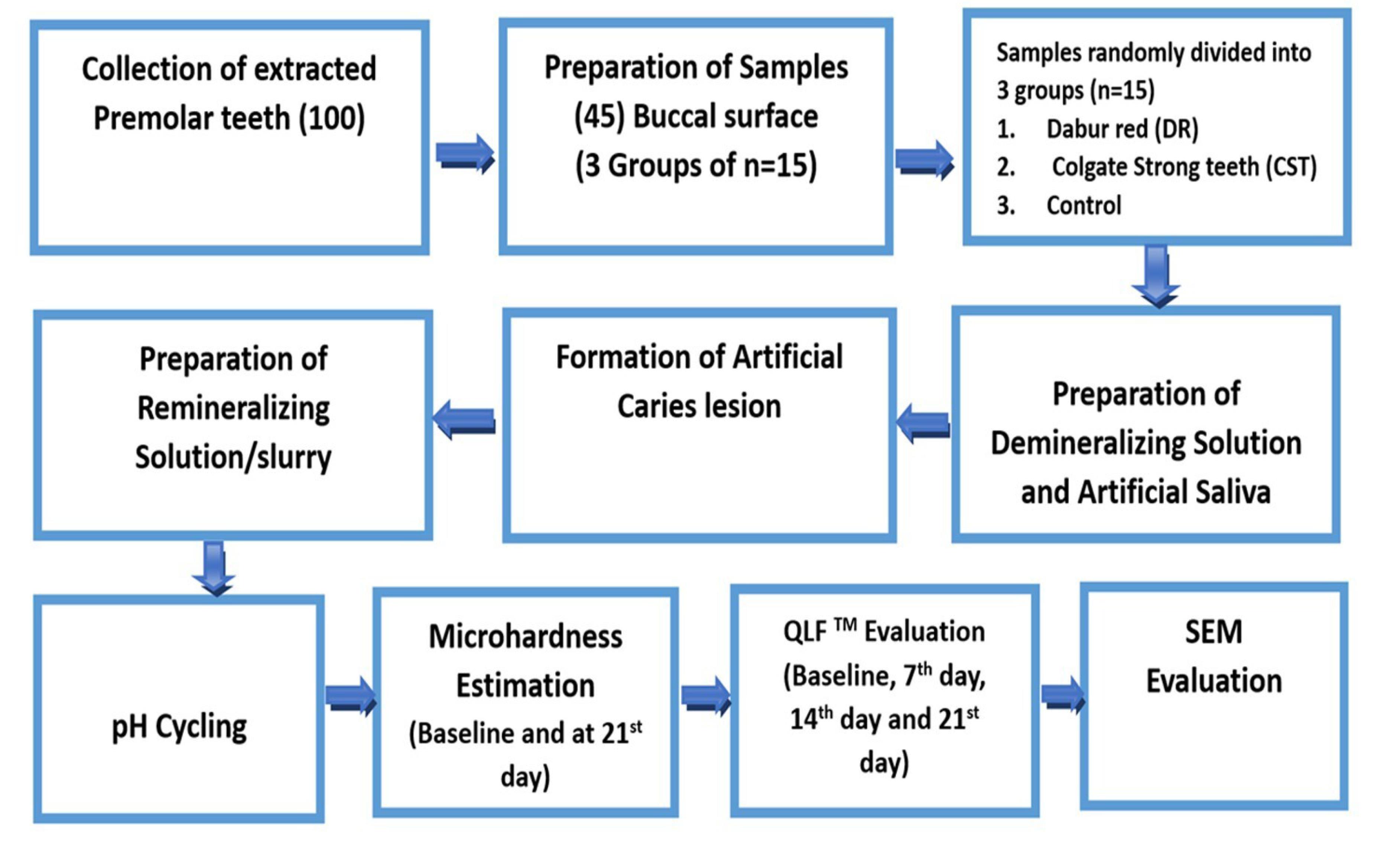
Figure 1.
Flowchart Representing the Step-by-step Method
.
Flowchart Representing the Step-by-step Method
Data Collection
One hundred freshly extracted maxillary pre-molars (indicated for orthodontic extraction) were collected and screened for inclusion in the study. The samples were provided by the Department of Oral and Maxillofacial Surgery. The collected samples were carefully cleansed of debris, calculus, and soft tissue before being evaluated for inclusion and exclusion criteria. After being cleaned in 0.1 M P buffer (pH = 7.4) and rinsed with deionized water, the samples were refrigerated in distilled water at 4°C until use. The inclusion criteria were caries-free teeth, therapeutically extracted teeth, and teeth with baseline zero fluorescence loss (QLF screening). On the other hand, the exclusion criteria were teeth with hypoplastic lesions, teeth with intrinsic stains, teeth with abrasion, erosion, and attrition, teeth with developmental anomalies, and teeth with restoration.
Sample Preparation
A total of 45 enamel samples were obtained by decoronating the teeth at the crown-root junction and further sectioning the crown into two halves mesiodistally. A diamond disc (Diatech CH 9435, Swiss Dental Instruments) continuously irrigated with deionized water was used to section the teeth. The cut surface of the buccal half of the tooth specimens was then embedded 2 mm into autopolymerizing polymethyl methacrylate acrylic (DPI, India) resin using PVC cylindrical molds of height 20 mm and diameter 20 mm (Figure 2A).
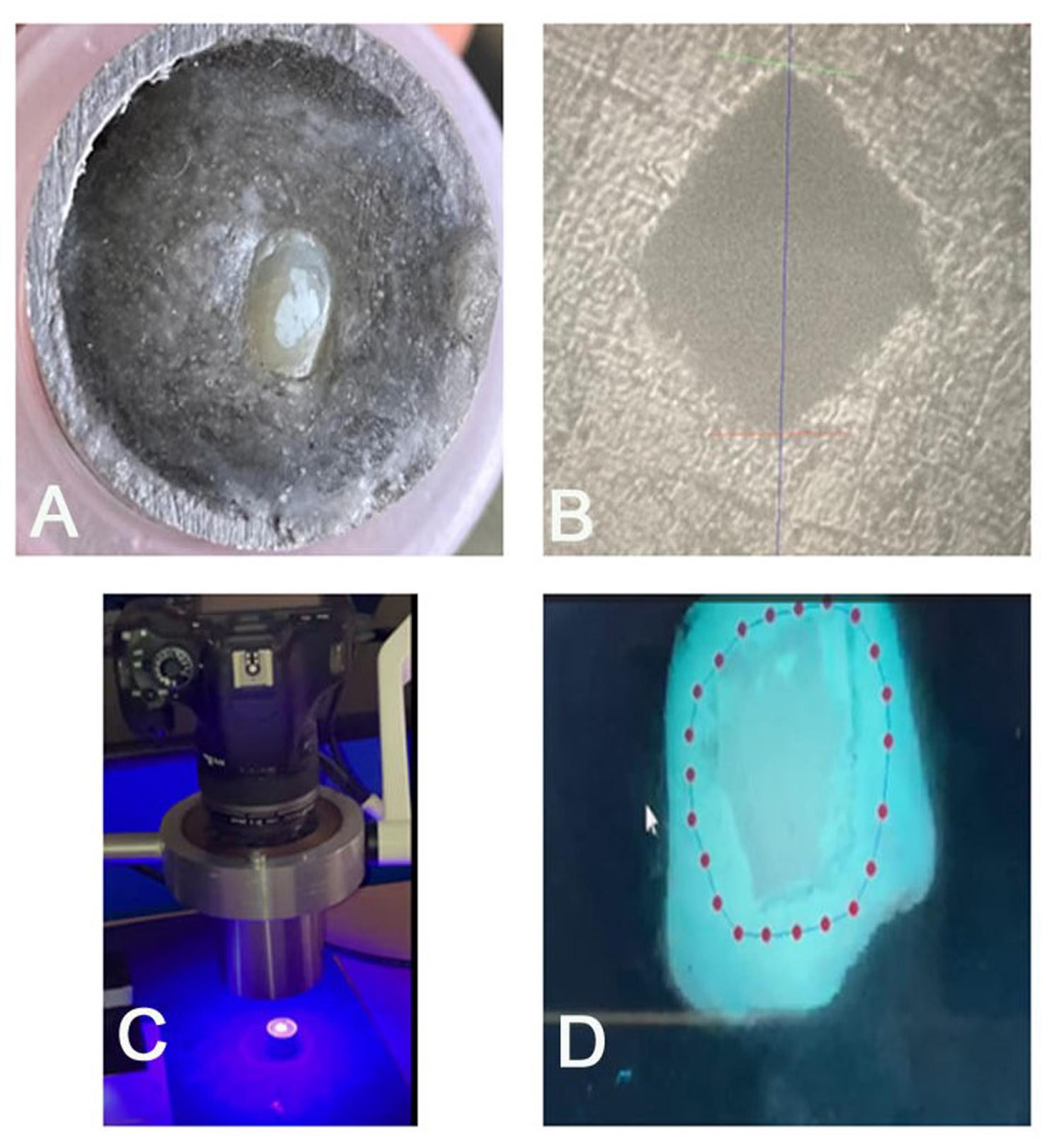
Figure 2.
(A) Specimen After Demineralization (Baseline), (B) Microhardness Test, Indentation, (C) QLF Device [QLF-D BiluminatorTM (Inspektor Research Systems BV, Amsterdam, The Netherlands)], and (D) Evaluation of QLF-D Images by QLF-D software. Note. QLF: Quantitative light-induced fluorescence system
.
(A) Specimen After Demineralization (Baseline), (B) Microhardness Test, Indentation, (C) QLF Device [QLF-D BiluminatorTM (Inspektor Research Systems BV, Amsterdam, The Netherlands)], and (D) Evaluation of QLF-D Images by QLF-D software. Note. QLF: Quantitative light-induced fluorescence system
The enamel samples were ground with 400-, 600-, and 1200-grit silicon carbide abrasive paper (Wolcut Abrasives, Delhi, India) under water irrigation to obtain flat surfaces. The specimens were polished using felt paper (3 M, USA), dampened with 0.5 μm diamond polishing paste (Ultradent Products, South Jordan, USA), and kept in distilled water until use.
The samples’ surface was covered with an adhesive polyvinyl tape measuring 2 × 2 mm, and a transparent acid-resistant nail varnish (Lakme, India) was then applied. The adhesive tape was taken off when the varnish had dried out, leaving a 2 × 2 mm window (Area of Interest) on the enamel surface.
Under a dental operating microscope (PICO, Zeiss, Germany), the region of interest was cleaned with a micro-brush. The samples were then inspected both clinically and digitally using QLF to ensure there were no apparent white spots, stained areas, or enamel malformations. New samples that satisfied the inclusion requirements were used to replace any damaged samples.
Formation of Artificial Caries Lesion
Demineralization was performed on each sample in order to produce artificial caries, or white spot lesions, in vitro. The literature suggests the use of a demineralization solution (pH = 5.0) for a 48-hour demineralization cycle (9,18,19). To standardize the demineralization process, the process was monitored round the clock using QLFTM, and the demineralization process was stopped when a sample achieved an adequate ΔF value (% loss of fluorescence related to lesion depth). Each sample was kept separately in sterile plastic vials (40 mL capacity) filled with 10 mL of the demineralizing solution. All samples were kept in an incubator that was kept at 37 °C. A fresh demineralizing solution was made every day.
Preparation of the Demineralizing Solution
The demineralizing solution was prepared using the ten Cate and Duijsters protocol (18). The demineralizing solution was composed of 2.2 mM of Ca chloride, 2.2 mM of potassium dihydrogen orthophosphate, 0.05 mM of acetic acid, and distilled water with a pH of 4.5 adjusted with sodium hydroxide (50%).
Preparation of Artificial Saliva
Artificial saliva was made using the technique described by Sato et al (20). Ingredients making up the artificial saliva, including Ca chloride (1.1 mM), magnesium chloride (0.08 mM), sodium carbonate (3.27 mM), Ca chloride (1.1 mM), sulfuric acid (0.05 mM), sodium P (3.90 mM), sodium chloride (4.29 mM), potassium chloride (17.98 mM), and 50% sodium hydroxide, were used to modify the pH of distilled water, which was 7.2.
Preparation of the Remineralizing Solution or Slurry
The manufacturer’s instructions were followed for using the interventional agents. To replicate the expected level of dilution that occurs daily when dentifrice is used, the equivalent paste was diluted using artificial saliva at a ratio of 1:3.
pH Cycling
The study of pH cycling (Figure 3) was performed for 21 days for all the specimens in accordance with the modified protocol by Featherstone et al (21). The samples were submerged for 30 minutes and split into two 15-minute intervals in 10 mL of a freshly made demineralizing solution. Between the two demineralization steps, the samples were washed in deionized water and immersed in 10 mL of freshly prepared artificial saliva for 6 hours.
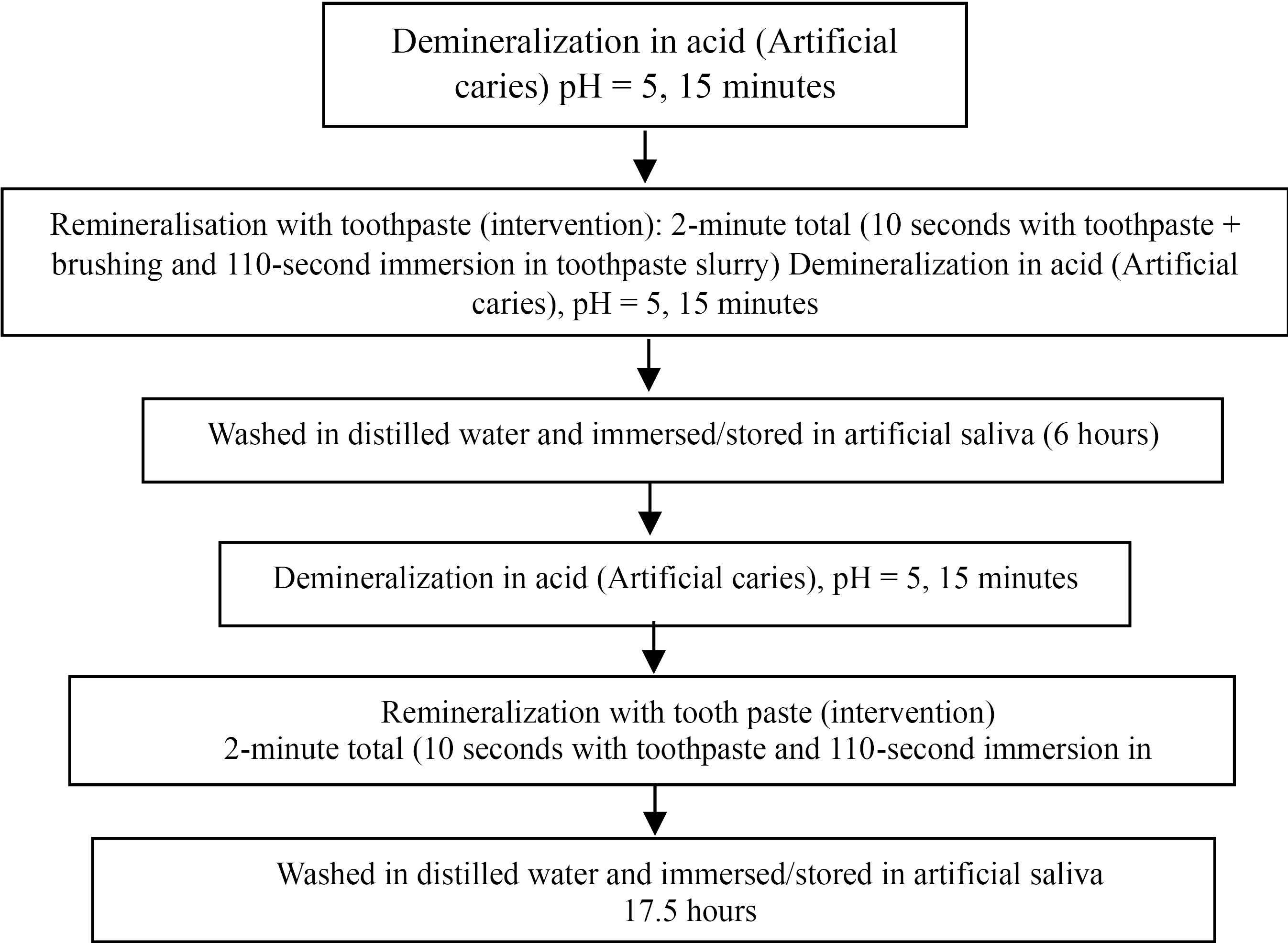
Figure 3.
pH Cycle Flowchart
.
pH Cycle Flowchart
A single, trained operator treated the samples using the appropriate interventional agents in accordance with the manufacturer’s guidelines. Then, the samples were stored in artificial saliva for 17.5 hours (overnight) at 37 °C in an incubator. This process was performed for 21 days.
Every specimen underwent QLFTM imaging and white spot analysis on the seventh, fourteenth, and twenty-first days of the pH cycling (Figures 2C-D). Every specimen underwent testing for VMH at baseline (before pH cycling) and following 21 days of pH cycling.
Microhardness Estimation
All the samples were evaluated for surface MH at baseline and after pH cycling (at the end of 21 days). The MH of the specimens was evaluated using a Vickers hardness testing machine (VHM machine). With a load of 100 g and a dwell duration of 10 seconds, the Vickers’ diamond indenter was gently applied to the test specimen using hydraulic damping. This produced a diamond pyramidal indentation, which was measured at 50X magnification using a digital scale (Figure 2B). Using a Vickers index unit formula, the computer automatically determined the surface MH; readings were generated as a Vickers hardness number (VHN). Each sample was indented three times, and the mean of the three readings was taken as the VHN of the specimen.
Quantitative Light-Induced FluorescenceTM Evaluation
To ensure that samples and the QLFTM camera were positioned consistently, a specially made jig was used to capture QLF images. The QLF-D BiluminatorTM (Inspektor Research Systems BV, Amsterdam, the Netherlands) was utilized by a single qualified examiner to capture QLFTM pictures. The device is made up of a customized 60 mm macro lens attached to a single-lens reflex camera, four high-power white LEDs, and twelve powerful blue LEDs. BiluminatorTM tubes focus light on the intended region of interest while accounting for ambient light. QLFTM and white-light digital images were taken from the buccal aspects of the specimens in a class 1 ASA darkroom condition. For white light photos, the shutter speed was 1/30 second, the aperture value was 20.0, and the ISO speed was 1600; for blue light photos, the shutter speed was 1/10 second, the aperture value was 10.0, and the ISO speed was 1600 (Figure 2C).
With the use of a specially made jig, the specimen and QLF-D were kept at a distance of 1 cm and an angle of 90°. The captured images (QLF-D) were evaluated by dedicated software (QA2 version 1.26, Inspektor Research Systems BV, Amsterdam, the Netherlands).
For every image, white spot lesion analysis was performed using masking, reconstruction, and blurring techniques (Figure 2D). ∆F (percentage fluorescence loss with respect to the fluorescence of sound tooth tissue related to lesion depth.), ∆Fmax (maximum percentage fluorescence loss with respect to the fluorescence of sound tooth tissue related to lesion depth), and ∆Q (percentage fluorescence loss with respect to the fluorescence of sound tissue times the area, related to lesion volume) were automatically computed as the mineral loss. The samples were rejected for any baseline fluorescence loss (∆F, less than -5%) and were replaced with new samples. The QLF analysis was performed at four intervals, namely, baseline, 7 days, 14 days, and 21 days (Figure 4).
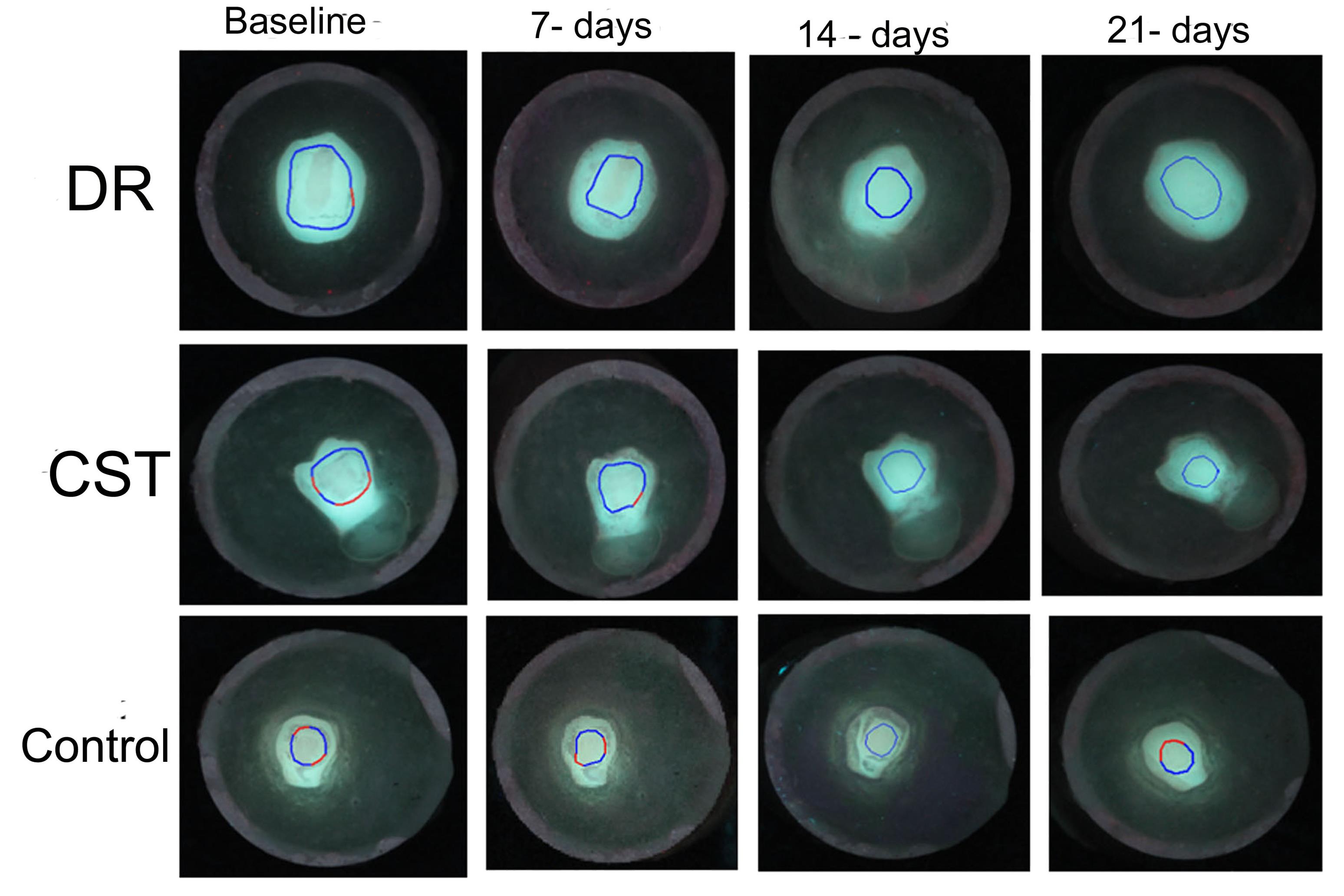
Figure 4.
QLF Images for Test (DR and CST) and Control Groups at Various Intervals Evaluated by QLF Software. Note. QLF: Quantitative light-induced fluorescence system; DR: Dabur Red; CST: Colgate strong teeth
.
QLF Images for Test (DR and CST) and Control Groups at Various Intervals Evaluated by QLF Software. Note. QLF: Quantitative light-induced fluorescence system; DR: Dabur Red; CST: Colgate strong teeth
Scanning Electron Microscopy Evaluation
To compare morphological variations (surface change) between different groups, one specimen from each group was randomly selected for SEM examination. The specimen was coated with Au/Pd in an SPI-MODULETM high-resolution sputter coater, mounted on the aluminum holder stubs using double-adhesive carbon tape, and inspected at 15 kV using an SEM (EVO® LS15, Carl Zeiss Microscopy GmbH, Goettingen, Germany).
Initially, the whole buccal surface of the sample was focused under the SEM’s center beam at a magnification of 1–10X. Pictures were acquired with a magnification of 2000x.
Statistical Analysis
The data were tabulated and statistically analyzed using SPSS software (version 22.0). In addition, the data were analyzed for normality using the Shapiro-Wilk test, which revealed the normal distribution of the data. A repeated-measures ANOVA was used for intragroup comparisons, and a one-way ANOVA with Tukey’s post-hoc was employed for intergroup comparisons. A P value < 0.05 was considered statistically significant.
Results
Microhardness (Vickers Microhardness)
In all three groups, there was a substantial rise in the mean VHN from the baseline to the 21st day of pH cycling (t-test, P < 0.05, Table 1). At 21-day MH analysis, the intergroup comparison (ANOVA) showed a statistically significant difference between the groups (P < 0.05). A significant difference was seen between the control and test groups. However, no significance was noted between the DR and CST (Tukey’s honest significant difference test).
Table 1.
Mean (SD), Mean Difference, and P values of Micro-hardness in VHN
|
Groups
|
Baseline MH (SD)
|
21-day MH (SD)
|
Mean Difference (SD)
|
P-value (Paired Sample t Test)
|
| DR |
40.47 (20.14) |
119.47 (18.73)a |
-78.99 (25.56) |
0.00 |
| CST |
41.91 (15.81) |
120.36 (30.83)a |
-78.45 (31.03) |
0.00 |
| Control |
36.41 (14.10) |
74.36 (22.76)c |
-37.95 (25.55) |
0.00 |
| ANOVA |
|
P < 0.05 |
|
|
SD: Standard deviation; VHN: Vickers hardness number; MH: Microhardness; DR: Dabur red; CST: Colgate strong teeth; ANOVA: Analysis of variance.
P < 0.05 is significant.
Quantitative Light-Induced Fluorescence Analysis
Loss of Fluorescence ΔF (%)
No statistically significant difference was found for baseline and 7-day intervals (Table 2). However, significant differences for 14 days and 21 days were observed among the groups. Comparing the groups at various intervals, a statistically significant difference was noted for each test group. For DR, the fluorescence loss at baseline and 7 days was statistically similar. Moreover, fluorescence loss at 14 days and 21 days was not significant. For CST, a significant difference in fluorescence loss was found between baseline and 7 days and between 7 days and 14 days. In the control group, a significant difference was detected between baseline and 7 days, 14 days, or 21 days. Hence, compared to the control, both the test products could remineralize the demineralized tooth structure to an optimum level in the in vitro condition.
Table 2.
Mean (SD) Loss of Fluorescence or ΔF (%) *
|
Interval
|
DR
|
CST
|
Control
|
Group Comparison**
|
| Baseline |
-8.27 (1.69)Aa |
-8.51 (1.35)Aa |
-8.37 (1.54)Aa |
P= 0.911 |
| 7 days |
-6.56 (1.39)Aa |
-6.54 (0.83)Ab |
-6.86 (0.9)Ab |
P= 0.533 |
| 14 days |
-2.46 (3.14)Ab |
-1.92 (2.61)Ac |
-6.51 (2.03)Bb |
P= 0.00 |
| 21 days |
-2.38 (3.04)Ab |
-1.50 (2.59)Ac |
-7.00 (1.86)Bb |
P= 0.00 |
| Interval comparison*** |
P= 0.00 |
P= 0.00 |
P= 0.00 |
|
SD: Standard deviation; DR: Dabur red; CST: Colgate strong teeth.
*ΔF (%) represents a quantitative measurement of demineralization for a given tooth surface and is the average percentage of fluorescence loss relative to the fluorescence of sound tissue. Negative ΔF values indicate an increase in mineral loss. **Capital letter superscript compares the significance across various groups (Tukey B post hoc test). ***The small letter superscript compares the significance across various intervals for a given group (Tukey B post hoc test). P < 0.05 is significant.
ΔFmax (%)
No statistically significant difference was noted among the test groups at baseline, 7-, 14-, and 21-day intervals (Table 3). However, specimens in the control group had more lesion depth compared to the test groups at 14 days and 21 days, which was statistically significant.
Table 3.
Mean (SD) of Maximum Lesion Depth or ΔFmax (%) *
|
Interval
|
DR
|
CST
|
Control
|
Group Comparison**
|
| Baseline |
-17.99 (7.9)Aa |
-19.15 (6.13)Aa |
-17.08 (5.60)Aa |
P = 0.709
|
| 7 days |
-9.48 (3.65)Ab |
-9.4 (2.38)Ab |
-11.47 (3.70)Ab |
P = 0.124
|
| 14 days |
-3.38 (4.14)Ac |
-2.49 (3.23)Ac |
-10.88 (5.7)Bb |
P = 0.000
|
| 21 days |
-3.20 (4.17)Ac |
-1.76 (8.04)Ac |
-10.14 (5.47)Bb |
P = 0.000
|
| Interval comparison*** |
P= 0.00 |
P= 0.00 |
P= 0.00 |
|
SD: Standard deviation; DR: Dabur red, CST: Colgate strong teeth
*ΔFmax (%) represents the maximal lesion depth. Negative ΔFmax values indicate an increase in lesion depth. **Capital letter superscript compares the significance across various groups (Tukey B post hoc test). ***The small letter superscript compares the significance across various intervals for a given group (Tukey B post hoc test). P < 0.05 is significant.
Statistically significant differences were observed when comparing the groups at various intervals. For DR and CST, the lesion depth decreased from baseline to 7 days and 14 days, respectively (P < 0.05). However, no noticeable change was found in lesion depth from 14 days to 21 days (P > 0.05). In the control group, a significant lesion depth reduction was noted between baseline and 7 days only (P < 0.05), and no further changes were noted for 14 days or 21 days (P > 0.05).
Scanning Electron Microscopy Findings
SEM micrographs at × 2000 magnification of a representative sample from each group were obtained after 21 days of treatment (Figure 5). The control group’s SEM micrographs revealed an enamel demineralization morphology that included porosities, an uneven slit and roughness pattern, and other signs of little remineralization. The images for DR and CST demonstrated dense mineralization.
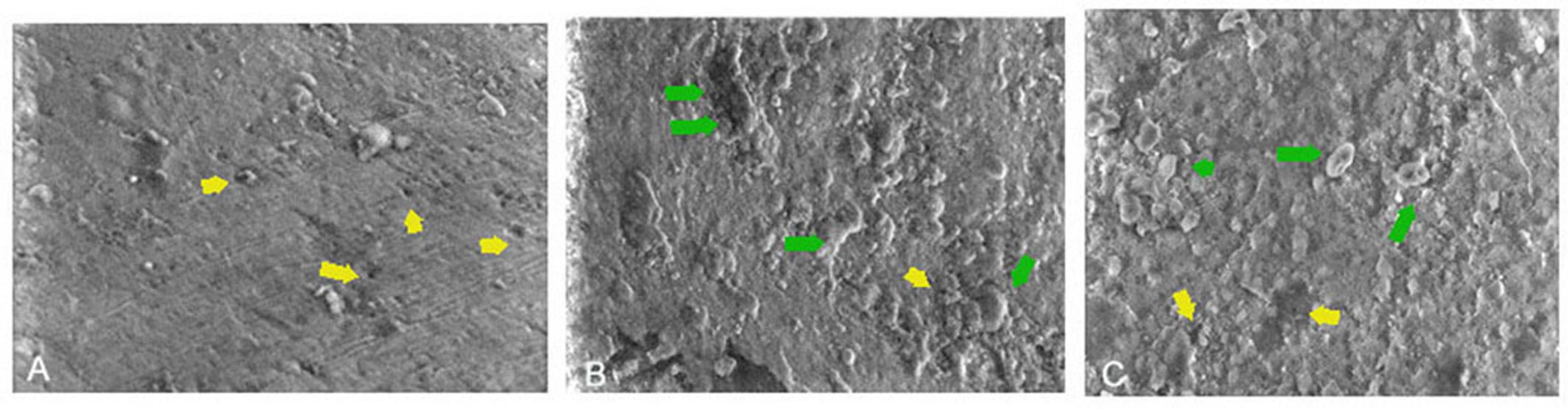
Figure 5.
SEM Images: (A) Control: Enamel Demineralization Morphology With Evidence of Porosities (Yellow Arrows), (B) Dabur Red: Uniform Pattern of Slits and Roughness, Indicative of Dense Remineralization (Green Arrow) and Minimal Porosities, and (C) Colgate Strong Teeth: An Irregular Pattern of Slits and Roughness, Indicative of Remineralization (Green Arrow) and Minimal Porosities. Note. SEM: Scanning electron microscopy
.
SEM Images: (A) Control: Enamel Demineralization Morphology With Evidence of Porosities (Yellow Arrows), (B) Dabur Red: Uniform Pattern of Slits and Roughness, Indicative of Dense Remineralization (Green Arrow) and Minimal Porosities, and (C) Colgate Strong Teeth: An Irregular Pattern of Slits and Roughness, Indicative of Remineralization (Green Arrow) and Minimal Porosities. Note. SEM: Scanning electron microscopy
Discussion
In recent years, there has been a significant increase in public interest in alternative health care, which includes the use of herbal health products (22). Plant-extractable dentifrices have become popular recently due to the expanding field of alternative medicine. In recent years, herbal ingredients have been present in oral care products, particularly in South Asian countries (23).
Many producers of dental hygiene products have added a variety of herbal ingredients to their products, claiming that these ingredients replicate the advantages of commercially available toothpaste, including their capacity to combat plaque, improve breath, prevent decay, and prevent gum disease. Although herbal toothpastes significantly prevent or reduce various oral conditions such as gingivitis and halitosis, their role in remineralizing tooth structure and caries prevention has not been extensively studied yet (24).
In the present study, freshly prepared artificial saliva was prepared by the method described by Sato et al (18). Throughout the remineralization regimen, the saliva was replaced every 24 hours to guarantee pH maintenance and ionic equilibrium. Test groups’ toothpaste slurries were used to simulate the dilution of the paste with saliva in the mouth (25).
The surface microhardness test is considered a well-established technique for assessing remineralization capacity in enamel, and surface hardness changes are appreciable after a few minutes of exposure to an acidic challenge. In addition, the indentations placed on enamel in the surface hardness test are not vulnerable to time-dependent changes in their morphology (26).
Various reports suggest changes in surface microhardness following the use of various herbal-based toothpastes (27,28). The findings of this investigation may be explained by the active herbal ingredients found in DR toothpaste, such as ginger and Garik powder.
Ginger has significant antibacterial and therapeutic properties, as demonstrated by earlier studies utilizing the rhizome of the plant (29). Studies on the remineralization potential of ginger have revealed that non-invasive treatment of artificially created early caries lesions with a ginger-containing solution resulted in enhanced remineralization and may have clinical benefits in caries management (30).
Furthermore, it is well known that dentifrices containing antimicrobial compounds are more effective in combating cariogenic bacteria (31). According to Mohan Kumar et al, toothpaste with several herbal constituents has a greater anti-cariogenic effect than toothpaste with fewer herbal ingredients (32). The DR also contains clove, which has anesthetic and antibacterial properties (33). The findings of a study confirmed that clove essential oil and its two major molecules prevent the demineralization brought on by the acidic beverage, or they might even encourage remineralization. The inhibition of the decalcification of the test compounds appears to be similar to the effect of fluoride treatment (34). The results for DR were statistically similar to those of a previous study, which concluded that DR had good remineralizing properties compared to other types of herbal toothpaste (24).
Notwithstanding their intrinsic differences, the findings of prior research demonstrated that QLFTM can be utilized as a tool for the identification and measurement of early white spot lesions in primary and permanent teeth without causing the sample’s structure to break down (35,36).
There is a clear correlation between fluorescence and the amount of mineral content. A drop in fluorescence indicates a loss of minerals, making it possible to quantify the loss of minerals (37). The ΔF and ΔF max for samples treated with CST and DR were similar at baseline and 7 days, but CST had less mineral loss at 14 days and 21 days. However, this was not statistically significant. However, the results of a study by Yadav et al represented that, compared to fluoridated toothpaste, minimal remineralization occurred with non-fluoridated toothpaste, and this was attributed to the low Ca (wt%) and P (wt%) ratios between the demineralizing and remineralizing groups, based on energy-dispersive X-ray analysis (25). In the present study, this Ca/P ratio was similar for all the groups. The reason for the similar Ca/P ratio in the control group could be attributed to the presence of fluoride in artificial saliva (25). This difference could be due to the difference in the substrate (dentin) and the experimental setup.
Fluoride-containing toothpaste remineralizes teeth by forming an acid-resistant, hypermineralized layer of fluorapatite-like material. Saliva and other topical sources provide Ca and P ions that are seeded to build the fluorapatite layer (38). For mineral deposition to take place inside the lesion’s body, there must be a sufficient level of Ca and P ions to break through the surface layer (39). Both test groups provided an adequate reservoir of Ca/P to facilitate remineralization.
The limitations of remineralization strategies include limited penetration depth and time, efficacy variation, material stability, and clinical evidence. The limitation of the present study is its in vitro nature, and future long-term clinical studies are required to corroborate the results.
Conclusion
Within the limitations of this in vitro study, it could be concluded that after 21 days of in vitro remineralization, evaluated by surface microhardness and QLF, both the DR and Colgate demonstrated similar remineralizing potential. In the remineralization of artificially carious lesions, it was found that herbal dentifrice was equally efficient as non-herbal dentifrices.
Acknowledgments
The authors would like to thank Vijnana Bhavana, the central instrumentation facility of the University of Mysore, for facilitating the SEM analysis for providing the study samples.
Authors’ Contribution
Conceptualization: Paras Mull Gehlot, Raghvendra Shanbhog, Jyoti Singh, Mahesh Katariya.
Data curation: Paras Mull Gehlot, Raghvendra Shanbhog, Jyoti Singh, Mahesh Katariya, Prasun Bandyopadhyay.
Formal analysis: Paras Mull Gehlot, Nandlal Bhojraj, Raghvendra Shanbhog, Mahesh Katariya, Prasun Bandyopadhyay.
Funding acquisition: Paras Mull Gehlot, Raghvendra Shanbhog, Nandlal Bhojraj.
Investigation: Paras Mull Gehlot, Raghvendra Shanbhog, Nandlal Bhojraj.
Methodology: Paras Mull Gehlot, Raghvendra Shanbhog, Jyoti Singh, Mahesh Katariya.
Project administration: Jyoti Singh, Nandlal Bhojraj, Mahesh Katariya, Prasun Bandyopadhyay.
Resources: Jyoti Singh, Prasun Bandyopadhyay, Mahesh Katariya, Paras Mull Gehlot, Nandlal Bhojraj.
Software: Paras Mull Gehlot, Raghvendra Shanbhog, Nandlal Bhojraj.
Supervision: Raghvendra Shanbhog, Nandlal Bhojraj, Jyoti Singh, Prasun Bandyopadhyay.
Validation: Raghvendra Shanbhog, Nandlal Bhojraj, Jyoti Singh, Mahesh Katariya.
Visualization: Paras Mull Gehlot, Raghvendra Shanbhog, Nandlal Bhojraj, Jyoti Singh, Mahesh Katariya, Prasun Bandyopadhyay.
Writing–original draft: Paras Mull Gehlot, Jyoti Singh, Raghvendra Shanbhog.
Writing–review & editing: Raghvendra Shanbhog, Nandlal Bhojraj, Jyoti Singh, Mahesh Katariya, Prasun Bandyopadhyay, Paras Mull Gehlot.
Competing Interests
This work was financially supported by the Dabur Research and Development Centre, Ghaziabad, Uttar Pradesh, India, as part of a consultancy project with JSS Dental College and Hospital, Mysuru, India. No authors from JSS Dental College received any consultancy fees.
Ethical Approval
The procedure protocol was reviewed and approved by the Institutional Research Ethics Committee of JSS Dental College and Hospital (IEC protocol No. 56/2022).
Funding
The study received financial support from theDabur Research and Development Centre (Dabur India, DCH/RD/22-23/1).
References
- Featherstone JD. Dental caries: a dynamic disease process. Aust Dent J 2008; 53(3):286-91. doi: 10.1111/j.1834-7819.2008.00064.x [Crossref] [ Google Scholar]
- Menon LU, Varma RB, Kumaran P, Xavier AM, Govinda BS, Kumar JS. Efficacy of a calcium sucrose phosphate-based toothpaste in elevating the level of calcium, phosphate ions in saliva and reducing plaque: a clinical trial. Contemp Clin Dent 2018; 9(2):151-7. doi: 10.4103/ccd.ccd_562_17 [Crossref] [ Google Scholar]
- Hara AT, Zero DT. The potential of saliva in protecting against dental erosion. Monogr Oral Sci 2014; 25:197-205. doi: 10.1159/000360372 [Crossref] [ Google Scholar]
- Rogerson MJ. The role of a calcium sucrose phosphate-calcium orthophosphate complex in the reduction of dental caries. Aust Dent J 1973; 18(3):160-6. doi: 10.1111/j.1834-7819.1973.tb03454.x [Crossref] [ Google Scholar]
- Karad A, Dhole P. Evaluation of remineralizing efficacy of calcium sucrose phosphate: a systematic review of in vitro studies. J Indian Orthod Soc 2019; 53(3):171-82. doi: 10.1177/0301574219862499 [Crossref] [ Google Scholar]
- Bhattacharjee S, Nath S, Bhattacharjee P, Chouhan M, Deb B. Efficacy of toothpastes on bacteria isolated from oral cavity. Int J Med Public Health 2018; 8(2):89-92. doi: 10.5530/ijmedph.2018.2.19 [Crossref] [ Google Scholar]
- Amoian B, Moghadamnia AA, Mazandarani M, Amoian MM, Mehrmanesh S. The effect of Calendula extract toothpaste on the plaque index and bleeding in gingivitis. Res J Med Plant 2010; 4(3):132-40. doi: 10.3923/rjmp.2010.132.140 [Crossref] [ Google Scholar]
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007; 369(9555):51-9. doi: 10.1016/s0140-6736(07)60031-2 [Crossref] [ Google Scholar]
- Pretty IA, Edgar WM, Higham SM. Detection of in vitro demineralization of primary teeth using quantitative light-induced fluorescence (QLF). Int J Paediatr Dent 2002; 12(3):158-67. doi: 10.1046/j.1365-263x.2002.00357.x [Crossref] [ Google Scholar]
- Ando M, van Der Veen MH, Schemehorn BR, Stookey GK. Comparative study to quantify demineralized enamel in deciduous and permanent teeth using laser- and light-induced fluorescence techniques. Caries Res 2001; 35(6):464-70. doi: 10.1159/000047491 [Crossref] [ Google Scholar]
- Bansal K, Balhara N, Marwaha M. Remineralizing efficacy of Calcarea fluorica tablets on the artificial carious enamel lesions using scanning electron microscope and surface microhardness testing: in vivo study. Indian J Dent Res 2014; 25(6):777-82. doi: 10.4103/0970-9290.152204 [Crossref] [ Google Scholar]
- Juntavee A, Juntavee N, Hirunmoon P. Remineralization potential of nanohydroxyapatite toothpaste compared with tricalcium phosphate and fluoride toothpaste on artificial carious lesions. Int J Dent 2021; 2021:5588832. doi: 10.1155/2021/5588832 [Crossref] [ Google Scholar]
- Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res 2008; 87(4):344-8. doi: 10.1177/154405910808700420 [Crossref] [ Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol 2014; 13(3):330-8. doi: 10.1016/s1474-4422(13)70278-3 [Crossref] [ Google Scholar]
- Martinez-Mier EA, Shone DB, Buckley CM, Ando M, Lippert F, Soto-Rojas AE. Relationship between enamel fluorosis severity and fluoride content. J Dent 2016; 46:42-6. doi: 10.1016/j.jdent.2016.01.007 [Crossref] [ Google Scholar]
- Veeramani R, Shanbhog R, Priyanka T, Bhojraj N. Remineralizing effect of calcium-sucrose-phosphate with and without fluoride on primary and permanent enamel: Microhardness and quantitative-light-induced-fluorescenceTM based in vitro study. Pediatr Dent J 2021; 31(1):51-9. doi: 10.1016/j.pdj.2020.12.001 [Crossref] [ Google Scholar]
- Suryani H, Gehlot PM, Manjunath MK. Evaluation of the remineralisation potential of bioactive glass, nanohydroxyapatite and casein phosphopeptide-amorphous calcium phosphate fluoride-based toothpastes on enamel erosion lesion - an ex vivo study. Indian J Dent Res 2020; 31(5):670-7. doi: 10.4103/ijdr.IJDR_735_17 [Crossref] [ Google Scholar]
- ten Cate JM, Duijsters PP. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res 1982; 16(3):201-10. doi: 10.1159/000260599 [Crossref] [ Google Scholar]
- Stookey GK, Featherstone JD, Rapozo-Hilo M, Schemehorn BR, Williams RA, Baker RA. The Featherstone laboratory pH cycling model: a prospective, multi-site validation exercise. Am J Dent 2011; 24(5):322-8. [ Google Scholar]
- Sato Y, Sato T, Niwa M, Aoki H. Precipitation of octacalcium phosphates on artificial enamel in artificial saliva. J Mater Sci Mater Med 2006; 17(11):1173-7. doi: 10.1007/s10856-006-0545-4 [Crossref] [ Google Scholar]
- Featherstone JD, Mellberg JR. Relative rates of progress of artificial carious lesions in bovine, ovine and human enamel. Caries Res 1981; 15(1):109-14. doi: 10.1159/000260508 [Crossref] [ Google Scholar]
- Vyas YK, Bhatnagar M, Sharma K. In vitro evaluation of antibacterial activity of an herbal dentifrice against Streptococcus mutans and Lactobacillus acidophilus. Indian J Dent Res 2008; 19(1):26-8. doi: 10.4103/0970-9290.38928 [Crossref] [ Google Scholar]
- Abhishek KN, Supreetha S, Sam G, Khan SN, Chaithanya KH, Abdul N. Effect of neem containing toothpaste on plaque and gingivitis--a randomized double blind clinical trial. J Contemp Dent Pract 2015; 16(11):880-3. doi: 10.5005/jp-journals-10024-1776 [Crossref] [ Google Scholar]
- Gomathi MS, Prasad SV, Iyer K, Jegadeson M, Indrapriyadhrshini K, Shrienitha DN. Remineralizing effect of commercially available two herbal dentifrices on human teeth-an in vitro evaluation. J Indian Assoc Public Health Dent 2023; 21(1):22-6. doi: 10.4103/jiaphd.jiaphd_171_21 [Crossref] [ Google Scholar]
- Yadav RK, Bharti D, Tikku AP, Verma P, Shakya VK, Pandey P. Comparative evaluation of remineralizing effect of fluoride and nonfluoride agents on artificially induced caries using different advanced imaging techniques. J Conserv Dent 2022; 25(1):26-31. doi: 10.4103/jcd.jcd_426_21 [Crossref] [ Google Scholar]
- Wongkhantee S, Patanapiradej V, Maneenut C, Tantbirojn D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J Dent 2006; 34(3):214-20. doi: 10.1016/j.jdent.2005.06.003 [Crossref] [ Google Scholar]
- Bilgin Göçmen G, Yanikoglu F, Tagtekin D, Stookey GK, Schemehorn BR, Hayran O. Effectiveness of some herbals on initial enamel caries lesion. Asian Pac J Trop Biomed 2016; 6(10):846-50. doi: 10.1016/j.apjtb.2016.08.005 [Crossref] [ Google Scholar]
- Kudva S, Prabhakar S, Pai V, Tegginamani A. Effects of garlic extract on salivary pH: a clinical study. Arch Orofac Sci 2012; 7(1):1-8. [ Google Scholar]
- Premkishore K, Umapathy T, Kathariya MD, Agrawal A, Kumar PP, Kallampilly G. Effect of honey and aqueous ginger extract against Streptococcus mutans isolated from extracted carious deciduous teeth. J Indian Acad Oral Med Radiol 2013; 25(4):265-7. [ Google Scholar]
- Celik ZC, Yavlal GO, Yanıkoglu F, Kargul B, Tagtekin D, Stookey GK. Do ginger extract, natural honey and bitter chocolate remineralize enamel surface as fluoride toothpastes? An in-vitro study. Niger J Clin Pract 2021; 24(9):1283-8. doi: 10.4103/njcp.njcp_683_20 [Crossref] [ Google Scholar]
- Sunitha J, Ananthalakshmi R, Jeeva JS, Jeddy N, Dhakshininamoorthy S, Muthu Meenakshi RM. Antimicrobial effect of herbal dentifrices: an in vitro study. J Pharm Bioallied Sci 2015; 7(Suppl 2):S628-31. doi: 10.4103/0975-7406.163575 [Crossref] [ Google Scholar]
- Mohankumar KP, Priya NK, Madhushankari GS. Anti cariogenic efficacy of herbal and conventional tooth pastes - a comparative in-vitro study. J Int Oral Health 2013; 5(2):8-13. [ Google Scholar]
- Alqareer A, Alyahya A, Andersson L. The effect of clove and benzocaine versus placebo as topical anesthetics. J Dent 2006; 34(10):747-50. doi: 10.1016/j.jdent.2006.01.009 [Crossref] [ Google Scholar]
- Marya CM, Satija G, J A, Nagpal R, Kapoor R, Ahmad A. In vitro inhibitory effect of clove essential oil and its two active principles on tooth decalcification by apple juice. Int J Dent 2012; 2012:759618. doi: 10.1155/2012/759618 [Crossref] [ Google Scholar]
- Kim HE, Kim BI. An in vitro comparison of quantitative light-induced fluorescence-digital and spectrophotometer on monitoring artificial white spot lesions. Photodiagnosis Photodyn Ther 2015; 12(3):378-84. doi: 10.1016/j.pdpdt.2015.06.006 [Crossref] [ Google Scholar]
- Veeramani R, Shanbhog R, Bhojraj N, Kaul S, Anoop NK. Evaluation of mineral loss in primary and permanent human enamel samples subjected to chemical demineralization by international caries detection and assessment system II and quantitative light-induced fluorescenceTM: an in vitro study. J Indian Soc Pedod Prev Dent 2020; 38(4):355-60. doi: 10.4103/jisppd.jisppd_181_20 [Crossref] [ Google Scholar]
- Dang MH, Jung JE, Lee DW, Song KY, Jeon JG. Recovery of acid production in Streptococcus mutans biofilms after short-term fluoride treatment. Caries Res 2016; 50(4):363-71. doi: 10.1159/000446408 [Crossref] [ Google Scholar]
- Shounia TY, Atwan S, Alabduljabbar R. Using silver diamine fluoride to arrest dental caries: a new approach in the US. J Dent Oral Biol 2017; 2(18):1105. [ Google Scholar]
- Mei ML, Ito L, Cao Y, Lo EC, Li QL, Chu CH. An ex vivo study of arrested primary teeth caries with silver diamine fluoride therapy. J Dent 2014; 42(4):395-402. doi: 10.1016/j.jdent.2013.12.007 [Crossref] [ Google Scholar]