Avicenna J Dent Res. 16(2):83-89.
doi: 10.34172/ajdr.1780
Original Article
Herpes Simplex Virus Type 1 Infection Up-regulates miR-221-5P in Oral Squamous Cell Carcinoma
Fatemeh Alipour 1  , Noushin Jalayer Naderi 2, *
, Noushin Jalayer Naderi 2, *  , Ronak Bakhtiari 3
, Ronak Bakhtiari 3 
Author information:
1Faculty of Dentistry, Shahed University, Tehran, Iran
2Department of Oral and Maxillofacial Pathology, Faculty of Dentistry, Shahed University, Tehran, Iran
3Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Background: Herpes simplex virus type 1 (HSV-1) infection contributes to oral carcinoma, but its carcinogenic role has not been elucidated yet. MicroRNAs (miRNAs) are involved in carcinogenesis. The aim of the present study was to examine the effect of HSV-1 infection on the expression of miR-29c, miR-221-5P, miR-21-5P, and miR-96-5P in patients with oral squamous cell carcinoma (OSSC).
Methods: Twenty-five cases of OSCC and 25 samples of normal oral mucosa were examined in this study. The SYBR real-time polymerase chain reaction (RT-PCR) was completed to confirm the presence of HSV-1. The expressions of miR-29c, miR-221-5P, miR-21-5P, and miR-96-5P were measured using RT-PCR.
Results: The expression of miR-221-5P was significantly higher in OSCC with HSV-1 infection compared to non-infected cases (P=0.007). The expressions of miR-29c, miR-21-5P, and miR-96-5P were not significantly different between OSCCs with HSV-1 infection and controls (P=0.27, P=0.66, and P=0.23, respectively).
Conclusion: HSV-1 infection had an up-regulation effect on miR-221-5P in OSCC. This finding proposes a novel mechanism for HSV-1 infection in the development of OSCC.
Keywords: Carcinoma, Herpes simplex virus, MicroRNA, Oral cancer
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Alipour F, Jalayer Naderi N, Bakhtiari R. Herpes simplex virus type 1 infection up-regulates miR-221-5P in oral squamous cell carcinoma. Avicenna J Dent Res. 2024; 16(2):83-89. doi:10.34172/ajdr.1780
Background
Oral cancer is one of the most life-threatening malignancies. Oral squamous cell carcinoma (OSCC) encompassesmore than 90% of oral malignant tumors (1). Different carcinogenic factors, such as tobacco alcohol,smoking, smokeless tobacco,occupational exposure, radiation, candida, and oncogenic viruses,are involved in the initiation and development of OSCC. Epstein-Barr virus (EBV), Herpes simplex virus (HSV), human papillomavirus (HPV), and retroviruses have been introduced as viral carcinogenic factors in OSCC development (2).
Viruses related to the occurrence of oral cancer are divided into two groups, including viruses that have a strong association with OSCC development, such as HPV and HSV. The second group belongs to viruses such as EBV, which have a negligible connection with oral cancer (3).Previous studies have shown the complementary RNA to HSV in more than 50% of OSCCs (4).Based on in vitro studies, HSV is mutagenic (5) and able to stimulate DNA synthesis (6).It has been suggested that HSV infection contributes to carcinogenesis through the activation and overexpression of c-myc and c-erb-B-1 (7).
MicroRNAs (miRNAs) are small RNAs with nucleotides with a length of 18–22 that are involved in messenger RNA degradation/translation (8). Studies demonstrate the important role of miRNAs in the pathogenesis of head and neck SCC (9-10).MiRNAs affect messenger RNAs and regulate gene expression (11).MiRNAs have a critical role in carcinogenesis as an oncogene or a tumor suppressor gene. Proliferation, differentiation, and genome stability are affected by microRNAs. Alterations in miRNA expression prompt the development of cancer (12).Theoverexpression of miR-221, miR-222, hsa-miR-29c, and hsa-miR-21 has been shown in oral cancer cells (13,14).
HSV has contributed to oral carcinoma initiation, but its carcinogenic role has not been elucidated so far (15).The findings of this study will help us understand the possible mechanisms of viral-induced OSCC. It was sought to examine the effect of HSV-1 infection on the expression of miR-29c, miR-221-5P, miR-21-5P, and miR-96-5P in OSSC. Based on our knowledge, this study is the first to focus on the expression of miR-29c, miR-221-5P, miR-21-5P, and miR-96-5P in HSV-infected oral cancer cells.
Materials and Methods
Tissue Samples
Twenty-five OSCC and 25 samples of normal oral mucosa were obtained from the archives of the Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences and the Pathology Department of Tehran University of Medical Sciences. All samples were formalin-fixed, paraffin-embedded sections from incisional biopsies. The selected samples were from the tongue and floor of the mouth. To consider the possible effects of hormones on OSCC development, only female samples entered the study. Adequate tumoral mass and the absence of necrosis/hemorrhage were the inclusion criteria. On the other hand, the exclusion criteria included chemotherapy and/or radiotherapy treatment. Marginal sections were not included in the examination (16).
Total DNA Extraction
Two 5 μm sections were prepared from each block and placed in a 2 mm microfuge tube. To remove paraffin, 1 mL of xylene was added to the tube holding the sample and then incubated in a shaker incubator for 30 minutes at a low speed of 55 °C. Then, the tube was centrifuged at 12 000 rpm for 2 minutes. The supernatant was removed, and the procedure was repeated 2 times. The tissue was washed twice with 100-degree ethanol. After the last wash, the ethanol was removed, and the tube with the tissue sediment inside was placed in the greenhouse until the ethanol evaporated completely.
Total DNA was extracted using the AmpliSens® DNA-sorb-B (MoBiTec GmbH) according to the manufacturer’s protocol. For this purpose, 0.1 mL of the sample and 300 μL of the warm lysis solutionwere placed in tubes and spined for 5 seconds, and then incubated at 65 °C for 5 minutes. Next, 400 μL of the precipitation solution was added and centrifuged at 12 000 rpm for 5 minutes. After removing the supernatant to a clean tube, 500 μL of the washing solution was added to each tube, spun thoroughly, and then centrifuged at 13 000 rpm for 2 minutes.
The processes of removing the supernatant to a clean tube, adding 200 μL of the washing solution to each tube, spinning thoroughly, and centrifuging at 13 000 rpm for 2 minutes were continued. After removing the supernatant completely, the tubes were incubated at 65°C for 5–10 minutes with open caps. Subsequently, 50 μL of the RNA buffer was added, and the tubes were vortexed and incubated at 65°C for 5 minutes. Finally, the tubes were centrifuged at 13 000 rpm for 1 minute.
Primers Designing
The primers (Table 1) for the HSV-1 strain RNA sequence were designed by using the Primer-BLAST online tool (17).
Table 1.
Primer Sequence for HSV-1
|
Type
|
Primer
|
Sequence (5'->3')
|
Length
|
Tm (°C)
|
GC%
|
Product Length
|
| HSV-1 |
Forward |
TATTGGTGCGATGGCGACAC |
20 |
61.09 |
55 |
143 bp |
|
|
Reverse |
CTTTCCGCATGTGGGCTCTC |
20 |
61.37 |
60 |
1 bp |
| RNaseP |
Forward |
AGA TTT GGA CCT GCG AGC G |
19 |
60.45 |
57.99 |
56 bp |
|
|
Reverse |
GAG CGG CTG TCT CCA CAA GT |
20 |
62.44 |
60 |
1 bp |
Note. Tm: Temperature; C: cytosine; G: guanine; HSV: Herpes simplex virus.
Real-Time Polymerase Chain Reaction
Real-time PCR was completed using primers designed with Primer-BLAST site software. According to the manufacturer’s protocol, 25µ of Master Mix Multiplex (Ampliqon) was used to test miRNA expression. The SYBR RT-PCR was set up in separate tubes for each viral agent. Inside each tube, a pair of primers for the target agent and the internal control gene were utilized, and the simultaneous reaction was set in 2 tubes. Finally, the results were confirmed based on melting curve analysis. B-actin was used as an internal control. PCR amplification and reading were achieved with the StepOne RT-PCR system under conditions of 4 minutes at 94 °C, followed by 40 cycles of 95 °C for 15 seconds, 55 °C for 55 seconds, 72 °C for 20 seconds, and 72 °C for 10 minutes (Figure 1).
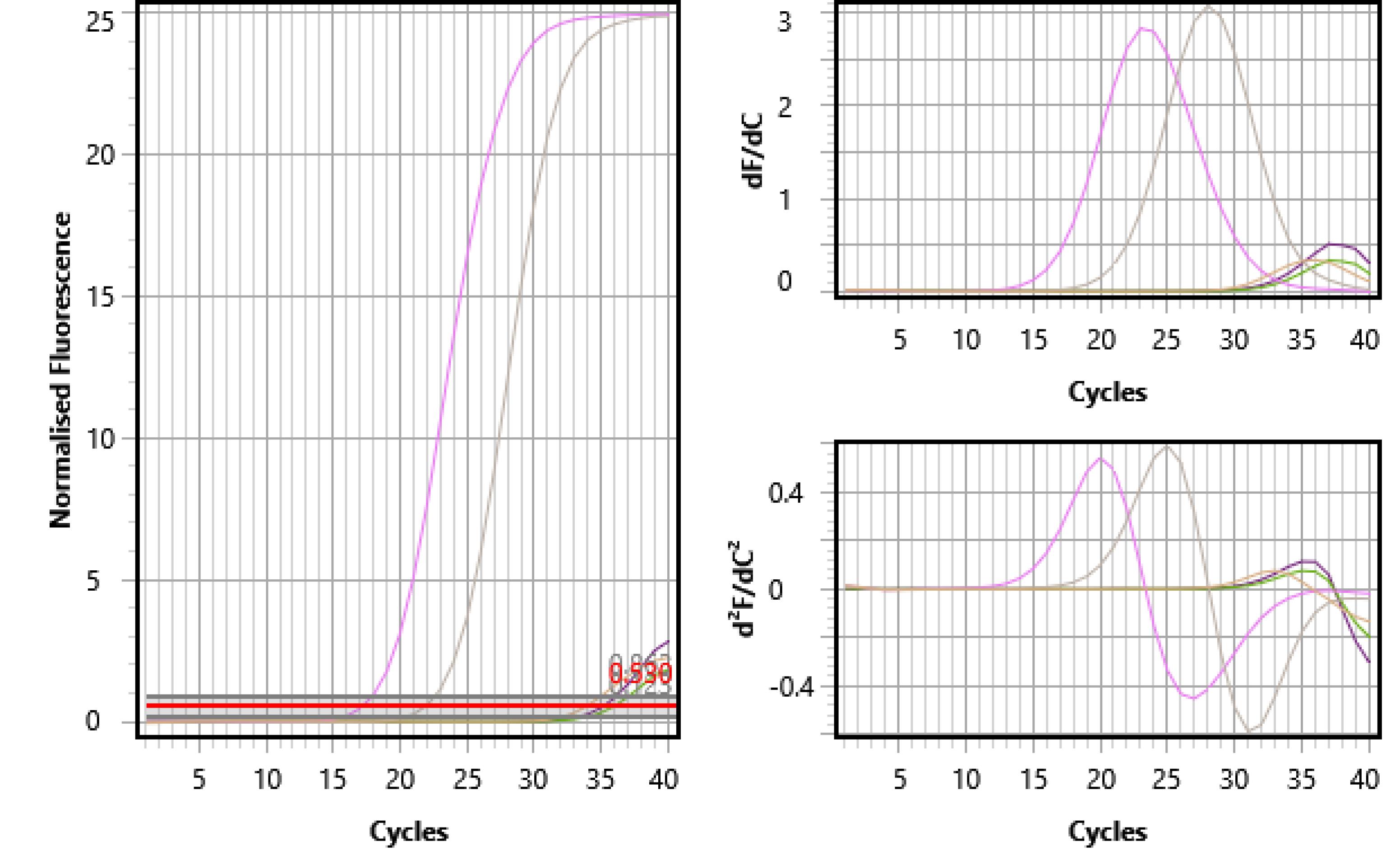
Figure 1.
Melting Curve Analysis for HSV-I Using SYBR Real-Time PCR. Note. HSV-I: Herpes simplex virus type 1; PCR: Polymerase chain reaction.
.
Melting Curve Analysis for HSV-I Using SYBR Real-Time PCR. Note. HSV-I: Herpes simplex virus type 1; PCR: Polymerase chain reaction.
Total RNA Extraction
About 20–25 mg of the homogenized sample tissue was placed in microtubes without nuclease. The homogenized tissue was washed with cold phosphate-buffered saline and then centrifuged at 3000 rpm for 2 minutes. In addition, 1 mL of trizole was added to the homogenized tissue, and after mixing, it was incubated for 5 minutes. Then, 250 μL of chloroform was added and vigorously shaken for 15 seconds. The microtube was placed at 4 °C for 15 minutes. The microtube was centrifuged for 20 minutes at 4 °C at 13 000 rpm. The blue phase was carefully removed from the microtube with a sampler and transferred to an additional microtube. Further, 500 µL of isopropanol was added, incubated on ice for 10 minutes, and then centrifuged at 13 000 rpm for 10 minutes at 4 °C. The supernatant was thrown out, and 1000 µL of 75% ethanol was added and mixed. The microtube was centrifuged for 5 minutes at 4 °C at 8000 rpm. To remove the ethanol, the microtube deposit was dried under a hood at room temperature for 10‒15 minutes. Furthermore, 50 µLof nuclease-free water was added to the dried sediment, and the microtube was placed at 55 °C for 15 minutes. To remove possible DNA contamination, the DNase I enzyme (Sinaclon Company, Iran) was used to extract the RNA. The microtube was incubated for 30 minutes at 37 °C, then 1 mL of ethylenediaminetetraacetic acid at 50 mM concentration was added, and the microtube was again placed at 65 °C for 10 minutes. The obtained samples were stored at -70 °C.
RNA Quality Assessment
RNA quality was assessed by optical density, and absorbance at 260 nm by the Nanodrop ND-1000 device (Nanodrop Technologies, Wilmington, DE).
Complementary DNA Synthesis
cDNA was completed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, K1622), according to the manufacturer’s guidelines; about 7 µL of RNA and 1 μL of the random hexamer primer up to 13 μL of diethylpyrocarbonate-treated water were incubated at 70 °C for 5 minutes and then placed on ice. Next, 4 μL of the 5x first-strand buffer and 1 μL of deoxynucleoside triphosphates were added and incubated at 25 °C for 5 minutes. After adding 1 μL of the reverse transcriptase enzyme, thermal cycling was achieved at 42 °C for 60 minutes and 70 °C for 10 minutes.
Real-Time Polymerase Chain Reaction
Gene expression was investigated by the relative qualification method. The RT-PCR test was performed using primers designed with MicroSEQ site software (Table 2).
Table 2.
Primer Sequence of Tested miRNAs
|
Tested miRNAs
|
miRNA Sequence
|
Primer
|
Sequence (5'->3')
|
| > hsa-miR-21-5p |
UAGCUUAUCAGACUGAUGUUGA |
Forward |
GCTTATCAGACTGATGTTGAGTCGT |
|
|
|
Reverse |
TCAACATCAGTCTGATAAGCTA |
|
|
|
Stem loop |
GTCGTGGTAGCTTATCAGACTGATGTTGACTGTTGAATCTCATGGCAA-
CACCAGTCGATGGGCTGTCTCTTCT |
| > hsa-miR-29c-5p |
UGACCGAUUUCUCCUGGUGUUC |
Forward |
GCTTATCAGACTGATGTTGAGTCGT |
|
|
|
Reverse |
GAACACCAGGAGAAATCGGTCA |
|
|
|
Stem loop |
GTCGTTGACCGATTTCTCCTGGTGTTCAGAGTCTGTTTTTGTCTAGCAC-
CATTTGAAATCGGTTATGATGTAGGGGGAGTTCT |
| > hsa-miR-96-5p |
UUUGGCACUAGCACAUUUUUGCU |
Forward |
GGCACTAGCACATTTTTGCTGTCGT |
|
|
|
Reverse |
TTTGGCACTAGCACATTTTTGCT |
|
|
|
Stem loop |
GTCGTGGCACTAGCACATTTTTGCTTGTGTCTCTCCGCTCTGAGCAAT-
CATGTGCAGTGCCAATATGGGAAATGCTT |
| > hsa- miR -221-5p |
ACCUGGCAUACAAUGUAGAUUU |
Forward |
CTGGCATACAATGUAGATTTGTCTG |
|
|
|
Reverse |
AAATCTACATTGTATGCCAG |
|
|
|
Stem loop |
GTCTGGGGCATGAACCTGGCATACAATGTAGATTTCTGTGTTCGTTAG-
GCAACAGCTACATTGTCTGCTGGGTTTCAGGCTACCTGGAAACAT-
GTTCTCTTTTCT |
The RT-PCR was conducted by the SYBR green method using the FIREPol® EvaGreen® qPCR Mix kit based on the instructions and temperature cycle; around 5 μL of template (cDNA), 12.5 µL of Master Mix, 4.5 µL of diethylpyrocarbonate water, and 1 µL of each primer (forward, reverse, and stem loop) were added to each vial. The reactions were completed along these lines, including 1 cycle at 50 °C for 30 minutes and then 1 cycle at 94 °C for 2 minutes, continuing by 40 cycles at 94 °C for 15 seconds, 55 °C for 30 seconds, and 72 °C for 30 seconds. The last cycle was at 72 °C for 10 minutes. The comparative CT method (ΔΔCt) was used to analyze differences in the expression of each group. The expression of the CT value of genes was calculated and compared with the expression of the RNaseP gene (Figure 2).
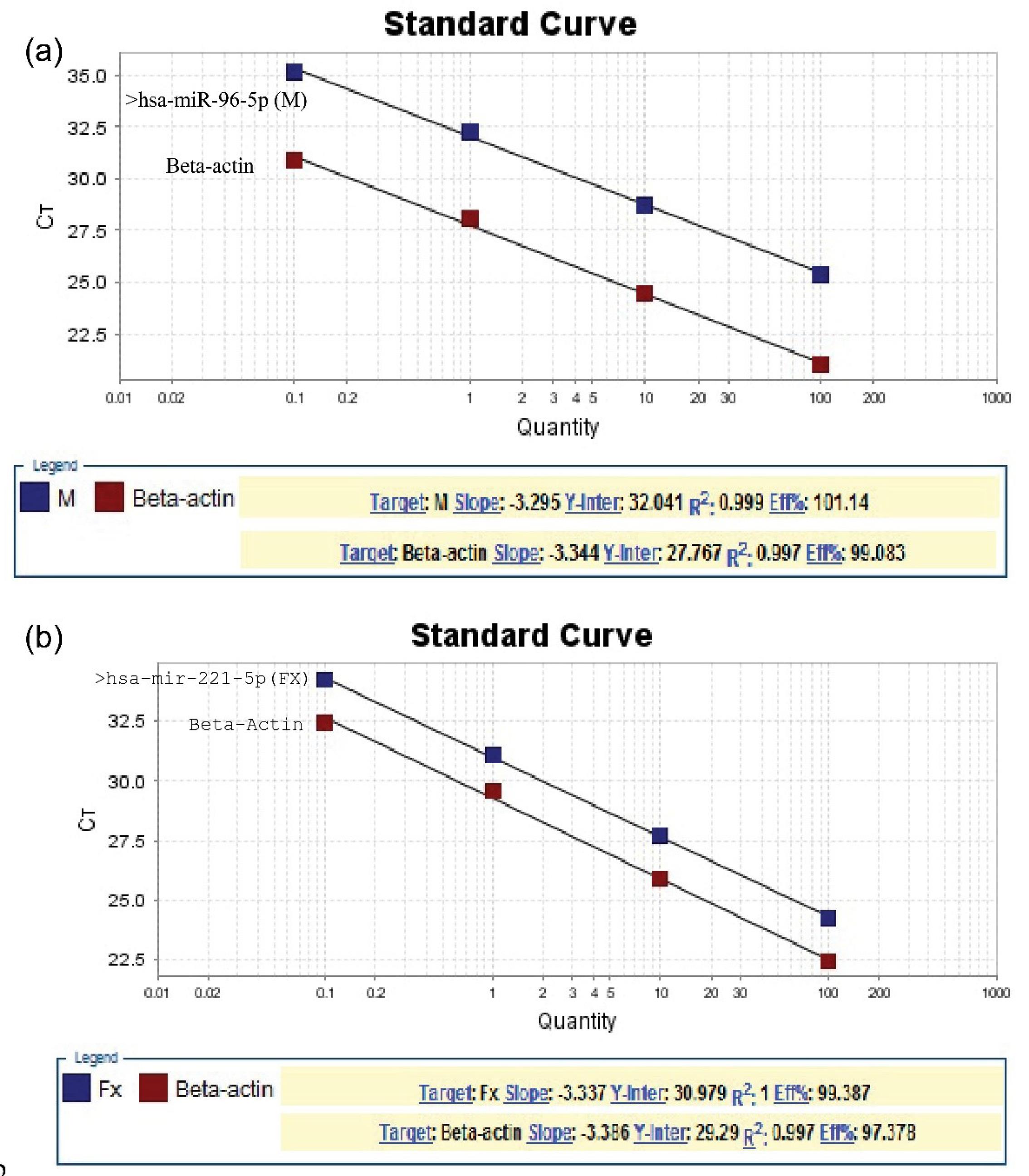
Figure 2.
Linear Regression Analysis of miR-96-5p: (a) and miR-221-5p and (b) Expression in Oral Squamous Revealed by the Real-Time PCR. Note. PCR: Polymerase chain reaction
.
Linear Regression Analysis of miR-96-5p: (a) and miR-221-5p and (b) Expression in Oral Squamous Revealed by the Real-Time PCR. Note. PCR: Polymerase chain reaction
Statistical Analysis
A two-tailed t-test was utilized to compare the difference in the mean expression of microRNAs (miR-29c, miR-221-5P, miR-21-5P, and miR-96-5P) between cases and controls at the P < 0.05 probability levelof the test. SPSS (version 22; IBM Company) was used for statistical analysis.
Results
The age range of OSCC cases was from 22 to 75 years, with a mean of 47.7 ± 12.5. In controls, the age ranged from 26 to 68 years, with a mean of 45 ± 8.4.
The expression of miR-96-5P and miR-221-5P was significantly higher in OSCCs than in controls (P = 0.005 and P < 0.0001, respectively). The expression of miR-21-5P and miR-29c was not significantly different between cases and controls (P = 0.18 and P = 0.11, respectively), the details of which are listed in Table 3.
Table 3.
MiRNA Expression in OSCC and Control Groups
|
MiRNAs
|
Groups
|
Number
|
Mean±SD
|
P
Value
|
| miR-21-5P |
OSCC |
25 |
20.99 ± 5.12 |
0.18 |
| Control |
25 |
23.31 ± 6.66 |
| miR-29C |
OSCC |
25 |
26.26 ± 4.57 |
0.11 |
| Control |
25 |
23.89 ± 5.47 |
| miR-96-5P |
OSCC |
25 |
25.89 ± 6.89 |
0.005 |
| Control |
25 |
20.28 ± 5.85 |
| miR-221-5P |
OSCC |
25 |
34.35 ± 4.26 |
< 0.0001 |
| Control |
25 |
21.97 ± 4.90 |
Note. OSCC: Oral squamous cell carcinoma; MiRNA: MicroRNA; SD: Standard deviation.
The expression of miR-221-5P was significantly higher in OSCC with HSV-1 infection compared to non-infected cases (P = 0.007). The expression of miR-29c, miR-21-5P, and miR-96-5P was not significantly different between OSCCs with HSV-1 infection and controls (P = 0.27, P = 0.66, and P = 0.23, respectively). The obtained data are summarized in Table 4.
Table 4.
MiRNA Expression in OCCs With and Without HSV-1 Infection
|
MiRNAs
|
Groups
|
Number
|
Mean±SD
|
P
Value
|
| miR-21-5P |
a + HSV-1 |
6 |
21.28 ± 3.473 |
0.66 |
|
b- HSV-1 |
18 |
22.44 ± 6.358 |
|
| miR-29C |
+ HSV-1 |
5 |
27.42 ± 5.139 |
0.27 |
| - HSV-1 |
19 |
24.70 ± 5.209 |
|
| miR-96-5P |
+ HSV-1 |
19 |
26.12 ± 3.364 |
0.23 |
| - HSV-1 |
25 |
22.21 ± 7.040 |
|
| miR-221-5P |
+ HSV-1 |
19 |
30.44 ± 4.386 |
0.007 |
| - HSV-1 |
25 |
23.49 ± 5.191 |
|
Note. SCC: Squamous cell carcinoma; MiRNA: MicroRNA; SD: Standard deviation.
Discussion
The results revealed that the expression of miR-96-5P and miR-221-5P was significantly higher in OSCC compared to controls. The expression of miR-221-5P was significantly higher in OSCC with HSV-1 infection in comparison to non-infected cases.
MiRNAs have been introduced in different cancer biogenesis processes, such as apoptosis, proliferation, and metastasis. MiRNAs may act as an oncogene or a tumor suppressor gene in different tumors. The expression of miR-221-5P was significantly higher in cases with OSCC than controls in this study, which is consistent with those of previous studies on prostate cancer (18), hypopharyngeal SCC (19), and renal cell carcinoma (20). It has been shown that miR-221-5p is involved in the proliferation of prostate cancer cells through the regulation of SOCS1 (18).Otherwise, the downregulation of miR-221-5p during the progression of prostate cancer expresses a tumor suppressive role (21). It seems that miR-221-5p modulates cell mechanisms through the interaction between different signaling pathways. Its function has not been elucidated in the OSCC progression and needs further research. The expression of miR-96-5P was significantly higher in OSCC, which is compatible with the results of previous studies in head and neck SCC (22), hepatocellular carcinoma (23), and ovarian cancer cells (24). Phosphatase and tensin homolog have been identified direct targets of miR-96-5p in head and neck SCC (22). The expression of miR-21-5P and miR-29c was not significantly different between OSCC and control groups in the present study, which contradicts the findings of previous studies, demonstrating the upregulation of miR-21-5P in OSCC (16,25).Wang et al reported that miR-29c overexpression inhibited the proliferation of OSCC cells (26).This is consistent with the result of this study. This controversy can be attributed to racial and genetic differences. In the studies related to the Iranian population, no study was found on miR-21-5P and miR-29c in OSCC. This finding needs further investigation.
HSV-1 is a dependent carcinogen in OSCC development and increases the risk of OSCC. HSV-1 has been identified in 15% of OSCC (27). It has been shown that viruses induce miR-146a in dengue virus infection (28), miR-130a in hepatitis C virus-infected hepatocytes (29), and miR-221 in HPV-16-positive cervical cancer cells (30). to intensify viral replication. The active or passive role of HSV-1 in the carcinogenesis of OSCC has not been evaluated yet. The expression of miR-221-5P was significantly higher in OSCC with HSV-1 infection compared to non-infected cases in the present study.Du et al found the negatively regulated IFN-β production in viral infection by miR-221 (31). Lu and Gu demonstrated the inhibition role of miR-221 in HPV 16 E1-E2 (30). The overexpression of miR-221 inhibited HSV-1-induced IFN-β expression and enhanced the infection of HSV-1 (31).It seems that by suppressing the immune response, miR-221 progresses tumor development. Based on these mechanisms, miR-221 may assist as a therapeutic target for HSV-1-infected OSCC. By virtue of this character, HSV-1 can have an effect on viral replication, host immunity, and cytokine expression. However, current knowledge about HSV-1 carcinogenesis is inadequate. In any case, identifying how HSV-1 affects the immune system will probably enable us to determine its carcinogenic effect (32).
The most important limitation of the present study was to obtain OSCC cases infected with HSV-1 in the females. Therefore, among the existing cases, many samples were excluded from the study because they did not meet the necessary inclusion criteria. The findings of this study confirmed that the expression of miR-221-5P was significantly higher in OSCC with HSV-1 infection. Hence, the expression of miRNAs can be a useful predictor for therapeutic and preventive purposes. It is suggested that the immune signaling pathway of miR-221-5P in HSV-1-infected OSCC be examined in future studies.
Conclusion
The findings of the present study revealed that the expression of miR-221-5P was significantly higher in OSCC with HSV-1 infection compared to non-infected cases. It has been hypothesized that HSV-1 has an up-regulation effect on miR-221-5P in OSCC. The expression of miR-29c, miR-21-5P, and miR-96-5P was not significantly different between OSCCs with HSV-1 infection and controls. Thus, based on the results of this study, it seems that miR-221 could affect the development of OSCC in HSV-infected cases. This finding proposes a mechanism for HSV infection in the development of OSCC. This can be a useful tool for therapeutic and preventive goals.
Acknowledgements
Nil.
Authors’ Contribution
Conceptualization: Noushin Jalayer Naderi and Ronak Bakhtiari.
Data Curation: Fatemeh Alipour and Ronak Bakhtiari.
Investigation: Fatemeh Alipour.
Methodology: Ronak Bakhtiari.
Project Administration: Noushin Jalayer Naderi.
Supervision: Noushin Jalayer Naderi.
Validation: Ronak Bakhtiari.
Visualization: Fatemeh Alipour, Noushin Jalayer Naderi, Ronak Bakhtiari.
Writing–original draft: Noushin Jalayer Naderi.
Writing–review & editing: Fatemeh Alipour, Noushin Jalayer Naderi, Ronak Bakhtiari.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The study was registered under the approval number IR.SHAHED.REC.1400.134.
Funding
The study was performed with the financial support of Shahed University.
References
- Ghafari R, Jalayer Naderi N, Emami Razavi A. A retrospective institutional study of histopathologic pattern of oral squamous cell carcinoma (OSCC) in Tehran, Iran during 2006-2015. J Res Med Sci 2019; 24:53. doi: 10.4103/jrms.JRMS_882_18 [Crossref] [ Google Scholar]
- Neville BW, Damm DD, Allen CM, Chi AC. Oral and Maxillofacial Pathology. 5th ed. India: Elsevier; 2024.
- Patil SP, Sodhi SJ, Tambe SD, Gada V, Vikhe D. Viruses in oral squamous cell carcinoma: a review. Pravara Med Rev 2016; 8(1):4-7. [ Google Scholar]
- Eglin RP, Scully C, Lehner T, Ward-Booth P, McGregor IA. Detection of RNA complementary to herpes simplex virus in human oral squamous cell carcinoma. Lancet 1983; 2(8353):766-8. doi: 10.1016/s0140-6736(83)92299-7 [Crossref] [ Google Scholar]
- Das CM, Zhang S, Shillitoe EJ. Expression of the mutagenic peptide of herpes simplex virus type 1 in virus-infected cells. Virus Res 1994; 34(2):97-114. doi: 10.1016/0168-1702(94)90093-0 [Crossref] [ Google Scholar]
- Steele C, Shillitoe EJ. Viruses and oral cancer. Crit Rev Oral Biol Med 1991; 2(2):153-75. doi: 10.1177/10454411910020020201 [Crossref] [ Google Scholar]
- Benner SE, Winn RJ, Lippman SM, Poland J, Hansen KS, Luna MA. Regression of oral leukoplakia with alpha-tocopherol: a community clinical oncology program chemoprevention study. J Natl Cancer Inst 1993; 85(1):44-7. doi: 10.1093/jnci/85.1.44 [Crossref] [ Google Scholar]
- Solomon MC, Radhakrishnan RA. MicroRNA's - the vibrant performers in the oral cancer scenario. Jpn Dent Sci Rev 2020; 56(1):85-9. doi: 10.1016/j.jdsr.2020.04.001 [Crossref] [ Google Scholar]
- Shojaei S, Menbari P, Jamshidi S, Taherkhani A. MicroRNA-based markers of oral tongue squamous cell carcinoma and buccal squamous cell carcinoma: a systems biology approach. Biochem Res Int 2023; 2023:5512894. doi: 10.1155/2023/5512894 [Crossref] [ Google Scholar]
- Taherkhani A, Shahmoradi Dehto S, Jamshidi S, Shojaei S. Pathogenesis and prognosis of primary oral squamous cell carcinoma based on microRNAs target genes: a systems biology approach. Genomics Inform 2022; 20(3):e27. doi: 10.5808/gi.22038 [Crossref] [ Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136(2):215-33. doi: 10.1016/j.cell.2009.01.002 [Crossref] [ Google Scholar]
- Wozniak M, Mielczarek A, Czyz M. miRNAs in melanoma: tumor suppressors and oncogenes with prognostic potential. Curr Med Chem 2016; 23(28):3136-53. doi: 10.2174/1389557516666160831164544 [Crossref] [ Google Scholar]
- Yang CJ, Shen WG, Liu CJ, Chen YW, Lu HH, Tsai MM. miR-221 and miR-222 expression increased the growth and tumorigenesis of oral carcinoma cells. J Oral Pathol Med 2011; 40(7):560-6. doi: 10.1111/j.1600-0714.2010.01005.x [Crossref] [ Google Scholar]
- Lopes CB, Magalhães LL, Teófilo CR, Alves A, Montenegro RC, Negrini M. Differential expression of hsa-miR-221, hsa-miR-21, hsa-miR-135b, and hsa-miR-29c suggests a field effect in oral cancer. BMC Cancer 2018; 18(1):721. doi: 10.1186/s12885-018-4631-z [Crossref] [ Google Scholar]
- Dabouian A, Tabibzadeh A, Salimi-Jeda A, Torkashvand S, Panahi M, Safarnezhad Tameshkel F. The molecular epidemiology of herpes simplex virus type 1 and 2 (HSV-1 and HSV-2) in head and neck cancer (HNC). Int J Cancer Manag 2020; 13(7):e105916. doi: 10.5812/ijcm.105916 [Crossref] [ Google Scholar]
- Mehterov N, Sacconi A, Pulito C, Vladimirov B, Haralanov G, Pazardjikliev D. A novel panel of clinically relevant miRNAs signature accurately differentiates oral cancer from normal mucosa. Front Oncol 2022; 12:1072579. doi: 10.3389/fonc.2022.1072579 [Crossref] [ Google Scholar]
- Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 2012; 13:134. doi: 10.1186/1471-2105-13-134 [Crossref] [ Google Scholar]
- Shao N, Ma G, Zhang J, Zhu W. miR-221-5p enhances cell proliferation and metastasis through post-transcriptional regulation of SOCS1 in human prostate cancer. BMC Urol 2018; 18(1):14. doi: 10.1186/s12894-018-0325-8 [Crossref] [ Google Scholar]
- Fan J, Wang C, Zhai X, Li J, Ju J, Zhu Y. lncRNA LEF1-AS1 acts as a novel biomarker and promotes hypopharyngeal squamous cell carcinoma progression and metastasis by targeting the miR-221-5p/GJA1 Axis. Dis Markers 2022; 2022:3881310. doi: 10.1155/2022/3881310 [Crossref] [ Google Scholar]
- Trilla-Fuertes L, Miranda N, Castellano D, López-Vacas R, Farfán Tello CA, de Velasco G. miRNA profiling in renal carcinoma suggest the existence of a group of pro-angionenic tumors in localized clear cell renal carcinoma. PLoS One 2020; 15(2):e0229075. doi: 10.1371/journal.pone.0229075 [Crossref] [ Google Scholar]
- Kiener M, Chen L, Krebs M, Grosjean J, Klima I, Kalogirou C. miR-221-5p regulates proliferation and migration in human prostate cancer cells and reduces tumor growth in vivo. BMC Cancer 2019; 19(1):627. doi: 10.1186/s12885-019-5819-6 [Crossref] [ Google Scholar]
- Vahabi M, Pulito C, Sacconi A, Donzelli S, D'Andrea M, Manciocco V. miR-96-5p targets PTEN expression affecting radio-chemosensitivity of HNSCC cells. J Exp Clin Cancer Res 2019; 38(1):141. doi: 10.1186/s13046-019-1119-x [Crossref] [ Google Scholar]
- Iwai N, Yasui K, Tomie A, Gen Y, Terasaki K, Kitaichi T. Oncogenic miR-96-5p inhibits apoptosis by targeting the caspase-9 gene in hepatocellular carcinoma. Int J Oncol 2018; 53(1):237-45. doi: 10.3892/ijo.2018.4369 [Crossref] [ Google Scholar]
- Liu B, Zhang J, Yang D. miR-96-5p promotes the proliferation and migration of ovarian cancer cells by suppressing Caveolae1. J Ovarian Res 2019; 12(1):57. doi: 10.1186/s13048-019-0533-1 [Crossref] [ Google Scholar]
- Aghiorghiesei O, Zanoaga O, Raduly L, Aghiorghiesei AI, Chiroi P, Trif A. Dysregulation of miR-21-5p, miR-93-5p, miR-200c-3p and miR-205-5p in oral squamous cell carcinoma: a potential biomarkers panel?. Curr Issues Mol Biol 2022; 44(4):1754-67. doi: 10.3390/cimb44040121 [Crossref] [ Google Scholar]
- Wang C, Wang Z, Zhang L, Lin X. MiR-29c inhibits the metastasis of oral squamous cell carcinoma and promotes its cell cycle arrest by targeting SERPINH1. Ann Transl Med 2021; 9(18):1423. doi: 10.21037/atm-21-3720 [Crossref] [ Google Scholar]
- Jalouli J, Jalouli MM, Sapkota D, Ibrahim SO, Larsson PA, Sand L. Human papilloma virus, herpes simplex virus and Epstein-Barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res 2012; 32(2):571-80. [ Google Scholar]
- Wu S, He L, Li Y, Wang T, Feng L, Jiang L. miR-146a facilitates replication of dengue virus by dampening interferon induction by targeting TRAF6. J Infect 2013; 67(4):329-41. doi: 10.1016/j.jinf.2013.05.003 [Crossref] [ Google Scholar]
- Bhanja Chowdhury J, Shrivastava S, Steele R, Di Bisceglie AM, Ray R, Ray RB. Hepatitis C virus infection modulates expression of interferon stimulatory gene IFITM1 by upregulating miR-130A. J Virol 2012; 86(18):10221-5. doi: 10.1128/jvi.00882-12 [Crossref] [ Google Scholar]
- Lu H, Gu X. MicroRNA-221 inhibits human papillomavirus 16 E1-E2 mediated DNA replication through activating SOCS1/Type I IFN signaling pathway. Int J Clin Exp Pathol 2019; 12(5):1518-28. [ Google Scholar]
- Du H, Cui S, Li Y, Yang G, Wang P, Fikrig E. MiR-221 negatively regulates innate anti-viral response. PLoS One 2018; 13(8):e0200385. doi: 10.1371/journal.pone.0200385 [Crossref] [ Google Scholar]
- Naqvi RA, Valverde A, Yadavalli T, Bobat FI, Capistrano KJ, Shukla D. Viral microRNAs in herpes simplex virus 1 pathobiology. Curr Pharm Des 2024; 30(9):649-65. doi: 10.2174/0113816128286469240129100313 [Crossref] [ Google Scholar]