Avicenna J Dent Res. 15(4):142-149.
doi: 10.34172/ajdr.1676
Original Article
Comparison of the Effect of Fluoride 0.2% and a Combined Mouthwash (Flavonoid Compounds and Fluoride 0.2%) Against Streptococcus mutans and Lactobacillus acidophilus: In Silico and In Vitro Study
Behin Omidi 1  , Yasin SarveAhrabi 1, *
, Yasin SarveAhrabi 1, *  , Sarina Nejati Khoei 1
, Sarina Nejati Khoei 1 
Author information:
1Department of Biology, Central Tehran Branch, Islamic Azad University, Tehran, Iran
Abstract
Background: Tooth decay is one of the most common health problems in the world. Nowadays, finding new compounds to prevent tooth decay is more necessary than ever. The purpose of this study was to compare the effects of fluoride 0.2% and mouthwash combined with flavonoid compounds against bacterial samples.
Methods: The crystal structures of glucansucrase from Streptococcus mutans and glucan-1, 6-alpha-glucosidase from Lactobacillus acidophilus were obtained from the Protein Data Bank. By using AutoDockTools (1.5.7), ligands and protein interactions were calculated and ready for AutoDock vina. The agar well diffusion and the minimum inhibitory concentration/minimum bactericidal concentration (MIC/MBC) methods were used to investigate the inhibitory effect of mouthwashes, and the results were obtained with SPSS software.
Results: Compounds eriocitrin and galangin showed the highest amount of H-bonds with amino acids against glucansucrase. In addition, catechin, eriocitrin, and isorhamnetin compounds demonstrated the highest amount of H-bonds with amino acids against glucan-1, 6-alpha-glucosidase. In vitro results revealed that groups a (Fluoride 0.2%+eriocitrin against S. mutans) and d (Fluoride 0.2%+eriocitrin against L. acidophilus) represented the most effect among all compounds, respectively (Inhibition zone=26±0.5 mm, MIC=250 µg/mL, MBC=500 µg/mL and inhibition zone=31±0.5 mm, MIC=125 µg/mL, MBC=250 µg/mL).
Conclusion: Fluoride 0.2% with eriocitrin was more effective in both methods (In silico and in vitro) compared to fluoride 0.2% due to its good inhibitory effect at different concentrations against S. mutans and L. acidophilus.
Keywords: Flavonoids, Streptococcus mutans, Lactobacillus acidophilus, Mouthwashes, Molecular docking simulation
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Omidi B, SarveAhrabi Y, Nejati Khoei S. Comparison of the effect of fluoride 0.2% And a combined mouthwash (flavonoid compounds and fluoride 0.2%) Against Streptococcus mutans and Lactobacillus acidophilus: in silico and in vitro study. Avicenna J Dent Res. 2023; 15(4):142-149. doi:10.34172/ajdr.1676
Background
Dental caries is one of the most common chronic and preventable diseases in the world (1). In general, caries is considered to be the result of the interaction between caries-causing oral flora and fermentable carbohydrates on tooth surfaces over time (2). According to several epidemiological studies, Streptococcus mutans is related to dental caries, and it seems that this gram-positive bacterium plays the main role in the initiation of caries (3). S. mutans affects tooth enamel by fermenting sucrose and producing lactic acid. In addition, this bacterium uses sucrose to make dental plaque. Dental plaque is made of dextran, which is a type of polysaccharide (4). S. mutansproduces three types of glucosyltransferases (GTF-I, GTF-SI, and GTF-S). The glucosyltransferase gene (gtfB) is encoded by GTF-I, is involved in the initial stages of glucan synthesis from sucrose, and is insoluble in water. This gene is one of the most important factors in the virulence of this bacterium (5-7). Glucan also plays a role in the formation of dental plaque and the firm adhesion of microorganisms to the tooth surface, resulting in the accumulation of acid and the beginning of decalcification on the surface of the enamel (8). Further, using advanced molecular techniques, several studies have shown that lactobacilli are located in the advanced areas of carious lesions, and probably these bacteria are related to dental caries (9). The reason for surface decay is the effect of acidic products resulting from bacterial fermentation, following the digestion of the protein matrix (matrix) by bacteria that cause tooth decay (10). Flavonoids are a group of naturally occurring polyphenolic compounds characterized by the flavan core and are one of the most common classes of compounds in fruits, vegetables, and plant-derived beverages (11). More than 8000 compounds with flavonoid structures have been identified. Together with carotenoids, they are responsible for the vibrant colors of fruits and vegetables. Some of the best-known flavonoids are quercetin and kaempferol. In plants, these compounds protect against UV rays, pathogens, and herbivores (12). Flavonoids are considered health-promoting and disease-preventing food supplements. Epidemiological, clinical, and animal studies show that flavonoids have antibacterial (13), antifungal (14), anticancer (15), and antioxidant (16) effects. According to the contents, this study focused on an in vitro and in silico comparison of the effects of fluoride 0.2% and a combination mouthwash (Flavonoid compounds and fluoride 0.2%) against S. mutans and L. acidophilus for use in mouthwashes and toothpastes.
Methods
In Silico: The crystal structure of glucansucrase from S. mutans (3AIE) and the structure of glucan-1, 6-alpha-glucosidase from L. acidophilus (4AIE) were received from https://www.rcsb.org (Protein Data Bank). The structures of flavonoids as ligands were received from https://pubchem.ncbi.nlm.nih.gov (PubChem).
In Vitro: All flavonoid compounds had been purchased from Sigma-Aldrich Company (Germany). The bacterial strains (S. mutans ATCC 35668 and L. acidophilus ATCC 4356) were organized by the Iranian Industrial Microorganisms Collection Center (Lyophilized). Microbiological assessments had been accomplished with the use of a Memmert- INC153T2T3 incubator.
In Silico
Ligand Preparation
The 2-dimensional (2D) structures of flavonoids (Table 1) as ligands (No. 1-10) were retrieved from ChemDraw Ultra 12.0.2.1076 (Figure 1) and saved in.pdb format. Use ChemBio3D Ultra 12.0 for the optimization of the ligands (17).
Table 1.
Structures Information of Flavonoid Compounds
|
No.
|
Name
|
PubChem CID
|
Molecular Formula
|
Molecular Weight (g/mol)
|
| 1 |
Acacetin |
5280442 |
C16H12O5 |
284.26 |
| 2 |
Apigenin |
5280443 |
C15H10O5 |
270.240 |
| 3 |
Caffeic acid |
689043 |
C9H8O4 |
180.16 |
| 4 |
Catechin |
9064 |
C15H14O6 |
290.271 |
| 5 |
Chrysin |
5281607 |
C15H10O4 |
254.241 |
| 6 |
Daidzein |
5281708 |
C15H10O4 |
254.23 |
| 7 |
Eriocitrin |
83489 |
C27H32O15 |
596.538 |
| 8 |
Fisetin |
5281614 |
C15H10O6 |
286.2363 |
| 9 |
Galangin |
5281616 |
C15H10O5 |
270.240 |
| 10 |
Isorhamnetin |
5281654 |
C16H12O7 |
316.26 |
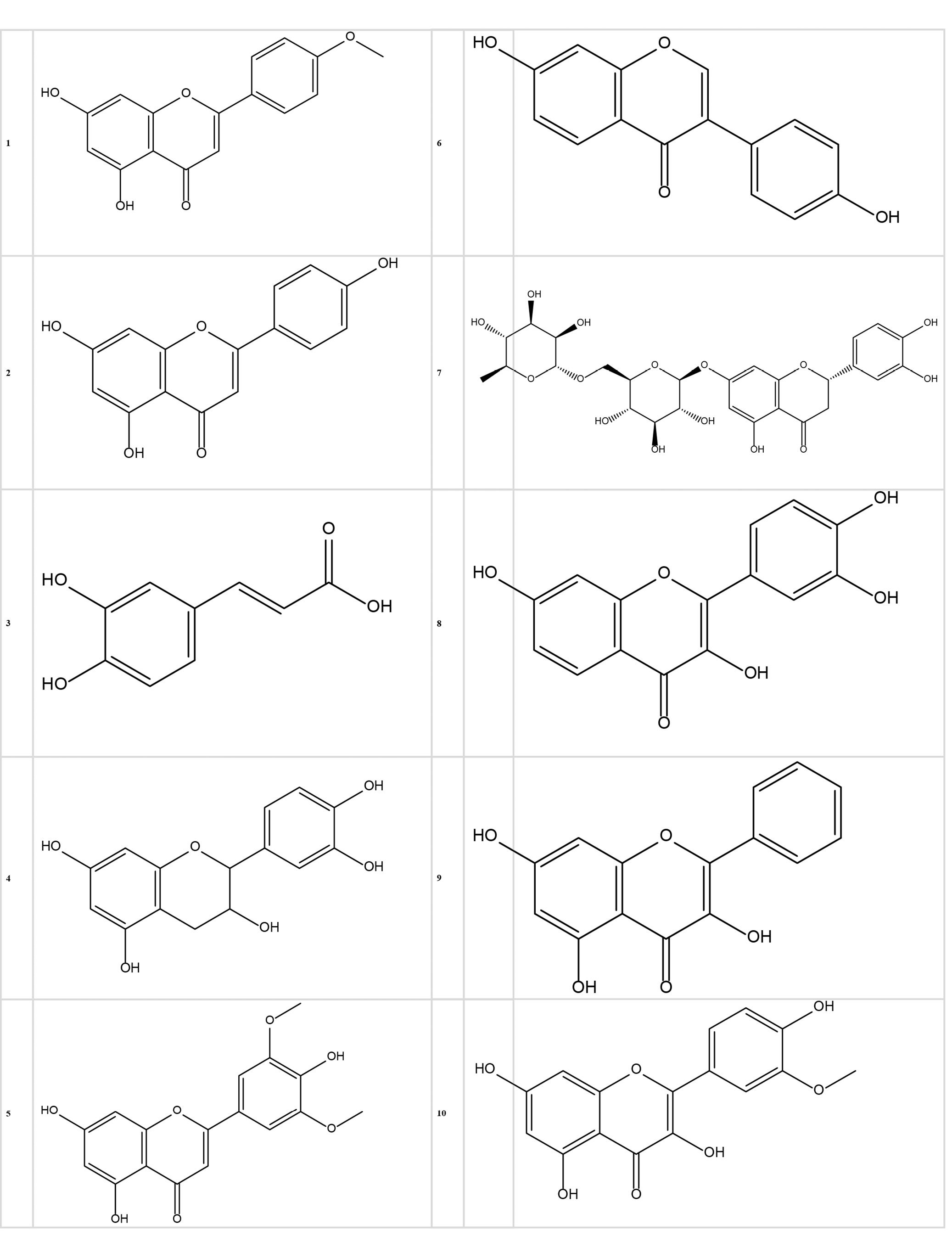
Figure 1.
2D Structures of Flavonoid Compounds (1-10). Note. 2D: 2-dimensional
.
2D Structures of Flavonoid Compounds (1-10). Note. 2D: 2-dimensional
Protein Preparation
The 2D structure of glucansucrase from Streptococcus mutans (PDB ID: 3AIE) and glucan-1, 6-alpha-glucosidase from Lactobacillus acidophilus (PDB ID: 4AIE) were obtained from the Protein Data Bank (https://www.rcsb.org/) with a resolution of 3.00 Å (Å: Angstrom) and in.pdb format. Subsequently, using Discovery Studio Client 4.5 software, the ligands and water molecules were separated from the main structures. Finally, the structures were prepared by adding polar hydrogens using AutoDockTools-1.5.6 software and saved in.pdbqt format.
Molecular Docking
Using the AutoDockTools (1.5.7) program, all polar hydrogen atoms have been introduced to the protein structure, and the partial masses of the ligands have been calculated and introduced with the Discovery Studio 4.5 Client program. The grid box for 3AIE was 118 × 118 × 118 dimensional, and dimensions have been additionally taken into consideration as X-center = 62.833, Y-center = 55.306, and Z-center = 3.139. Furthermore, the grid box for 4AIE was 94 × 94 × 94 dimensional, and dimensions have been additionally taken into account as X-center = 9.002, Y-center = 26.444, and Z-center = 27.248. The overall findings obtained through molecular docking were examined using AutoDockTools-1.5.6 software (18). To analyze the results in greater detail, the ligand-receptor complex corresponding to the conformation with the highest binding energy was prepared and assessed for each compounds using Discovery Studio Client 4.5 software (2D and 3D).
In Vitro
The bacteria that were prepared as lyophilized were inoculated in the broth medium. Brain heart infusion (BHI) agar/broth and Man–Rogosa–Sharpe (MRS) agar/broth were used for S. mutans and L. acidophilus., respectively. Next, the bacterial samples were kept for 48 hours in an anaerobic jar at 37 °C. Then, the bacteria suspension was prepared according to McFarland’s 0.5 standard.
Agar Well Diffusion Method
To perform this experiment, wells of 5 mm in diameter were created by a sterile pipette in culture media containing cultured bacterial suspension. Then, the wells were filled with samples (S. mutans wells: Fluoride 0.2% (control), fluoride 0.2% + eriocitrin (a), Fluoride 0.2% + galangin (b). L. acidophilus wells: Fluoride 0.2% (control), fluoride 0.2% + catechin (c), Fluoride 0.2% + eriocitrin (d), Fluoride 0.2% + isorhamnetin (e)). Next, the plates were put inside the incubator for 24 hours at 37 °C. It is worth noting that all the steps were performed near the flame and in a sterile environment (19). This experiment was repeated three times, and their mean was reported in the results.
Broth Dilution Method
To determine the minimum inhibitory concentration (MIC), a series of 9 tubes were used to test the different dilutions of each compound. It is noteworthy that the control sample was diluted in 9 separate tubes. The initial concentration of each compound was 2 mg/mL (2000 µg/mL), which was obtained by inserting 1 mL of the compounds into the first tube containing 1 mL of culture medium at a concentration of 1 mg/mL (1000 µg/mL). Different dilutions were obtained from tubes No. 1 (2 mg/mL) to No. 9 (0.007 mg/mL). To this end, 1 mL of the compounds in the first tube with a concentration of 2 mg/mL was diluted with 1 mL of culture medium in the second tube (BHI broth and MRS broth were utilized for S. mutans and L. acidophilus, respectively). In this way, 1 mL was removed from the first tube and added to the second tube, containing 1 mL. This was continued up to tube No. 9, then 1 mL was removed from the last tube and ejected, which eventually resulted in the half-dilution of the previous tube. Then, 50 μL of microbial suspension containing 1.5 × 108 bacteria were transferred to the tubes. All the test tubes were incubated (for 24 hours at 37 ̊C). After incubation, the tubes were tested for turbidity because of the bacterial growth. All tubes with no bacterial growth were sampled and cultured to determine the minimum bactericidal concentration (MBC) of the compounds. For this purpose, the tubes showing no bacterial growth were cultured on the culture medium (BHI agar and MRS agar were used for S. mutansand L. acidophilus, respectively). After incubation for 24 hours, the cultured plates were controlled for microbial growth. The lowest concentration of compounds in the relevant plates, exhibiting bacterial growth failure, was considered the MBC of those compounds (20).
Results
In Silico
All affinities of flavonoid compounds (1-10) against bacterial strains were calculated, the results of which are provided in Table 2. The affinity of all compounds was reported between -5.2 and -9.2 kcal/mol. Among these, compounds 7 (eriocitrin) and 9 (galangin) demonstrated the highest amount of H-bonds with amino acids against 3AIE; further, these compounds created the highest amount of variety and number of hydrogen bonds with amino acids such as asparagine, glutamine, aspartic acid, and glutamic acid with compound 7 (eriocitrin) and amino acids serine, tryptophan, and threonine with compound 9 (galangin). Compounds 4 (catechin), 7 (eriocitrin), and 10 (isorhamnetin) showed the highest amount of H-bonds with amino acids against 4AIE; moreover, these compounds created the highest amount of variety and number of hydrogen bonds with amino acids such as serine, glutamic acid, aspartic acid, lysine, and histidine with compound 4 (catechin), as well as amino acids aspartic acid, asparagine, leucine, tyrosine, proline, and serine with compound 7 (eriocitrin), along with amino acids arginine, asparagine, and aspartic acid with compound 10 (isorhamnetin). According to Figure 2, compound 7 (eriocitrin) has the ability to bind and inhibit both structures with an affinity of -8.6 for 3AIE and -9.2 for 4AIE. This compound against S. mutans (3AIE) can inhibit the structure by creating hydrogen bonds with amino acids glutamine (864 H), asparagine (625 H), aspartic acid (666 H), and glutamic acid (622 H). Additionally, against L. acidophilus (4AIE), it can inhibit the structure by creating hydrogen bonds with amino acids such as aspartic acid (327 H), asparagine (361 H and 326 H), leucine (285 H), tyrosine (530 H), proline (284 H), and serine (322 H).
Table 2.
Chem 3D and AutoDockVina Results of Compounds (1-10)
|
Compounds
|
Total Energy
(KCal/Mol)
|
Affinity ∆Gbind
(KCal/Mol)
|
H-Bonds
|
Pi-Pi
|
S.
Mutans
(3AIE)
|
L. acidophilus
(4AIE)
|
S.
Mutans
(3AIE)
|
L. acidophilus
(4AIE)
|
S.
Mutans
(3AIE)
|
L. acidophilus
(4AIE)
|
| Acacetin |
21.4567 |
-7.4 |
-8.4 |
PHE866
GLN864 |
ARG401
ARG212
ASP198 |
ALA865
MET614
LYS618 |
ASP316
TYR62
PHE162
TRP242
VAL199 |
| Apigenin |
14.4725 |
-7.3 |
-8.7 |
ASN481
ASP477 |
ARG212
GLU201 |
ASP480
LEU433
ALA478 |
ASP316
ARG401
GLU240
TRP242 |
| Caffeic acid |
20.9818 |
-5.2 |
-6.5 |
LEU395
SER397 |
SER3
SER261 |
VAL1074 |
PRO255 |
| Catechin |
-3.9469 |
-7.6 |
-8.9 |
ASP593
ASP477 |
SER253
GLU259
ASP234
LYS232
HIS258
|
GLN592
TYR916
ASP588 |
PRO255 |
| Chrysin |
15.6501 |
-6.7 |
-8.5 |
GLN592 |
- |
TYR916
ASP909
ASP588 |
LYS279
TRP242 |
| Daidzein |
27.1388 |
-6.5 |
-7.4 |
GLN864 |
GLN273 |
ASP666
LYS618
MET614 |
GLU240
TRP242 |
| Eriocitrin |
31.4286 |
-8.6 |
-9.2 |
ASN625
GLN864
ASP666
GLU622
|
ASN361
ASP327
ASN326
LEU285
TYR530
PRO284
SER322
|
LYS618 |
VAL398 |
| Fisetin |
23.3827 |
-7.4 |
-6.9 |
- |
ARG401
ASP198 |
TRP517 |
TRP242
VAL199
ASP316 |
| Galangin |
22.9427 |
-8.7 |
-7.0 |
SER1083
THR298
TRP299
|
- |
ALA246
TRP300 |
TRP242
GLU240
ASP316
ARG401
TYR62
ASP198 |
| Isorhamnetin |
27.6031 |
-7.4 |
-8.6 |
ASP909
GLU515 |
ARG212
ASN242
ASP316
ARG401
|
TRP517
ASP588
HIS587
TYR916
LEU433 |
PHE162
TRP242 |
Note. SER: Serine; VAL: Valine; TYR: Tyrosine; TRP: Tryptophan; PHE: Phenylalanine; HIS: Histidine; ARG: Arginine; ASN: Asparagine; GLN: Glutamine; PRO: Proline; ASP: Aspartic acid; THR: Threonine; LEU: Leucine; GLU: Glutamic acid; LYS: Lysine; GLY: Glycine. S. Mutans: Streptococcus mutans; L. acidophilus: Lactobacillus acidophilus; SD: Three dimensional.
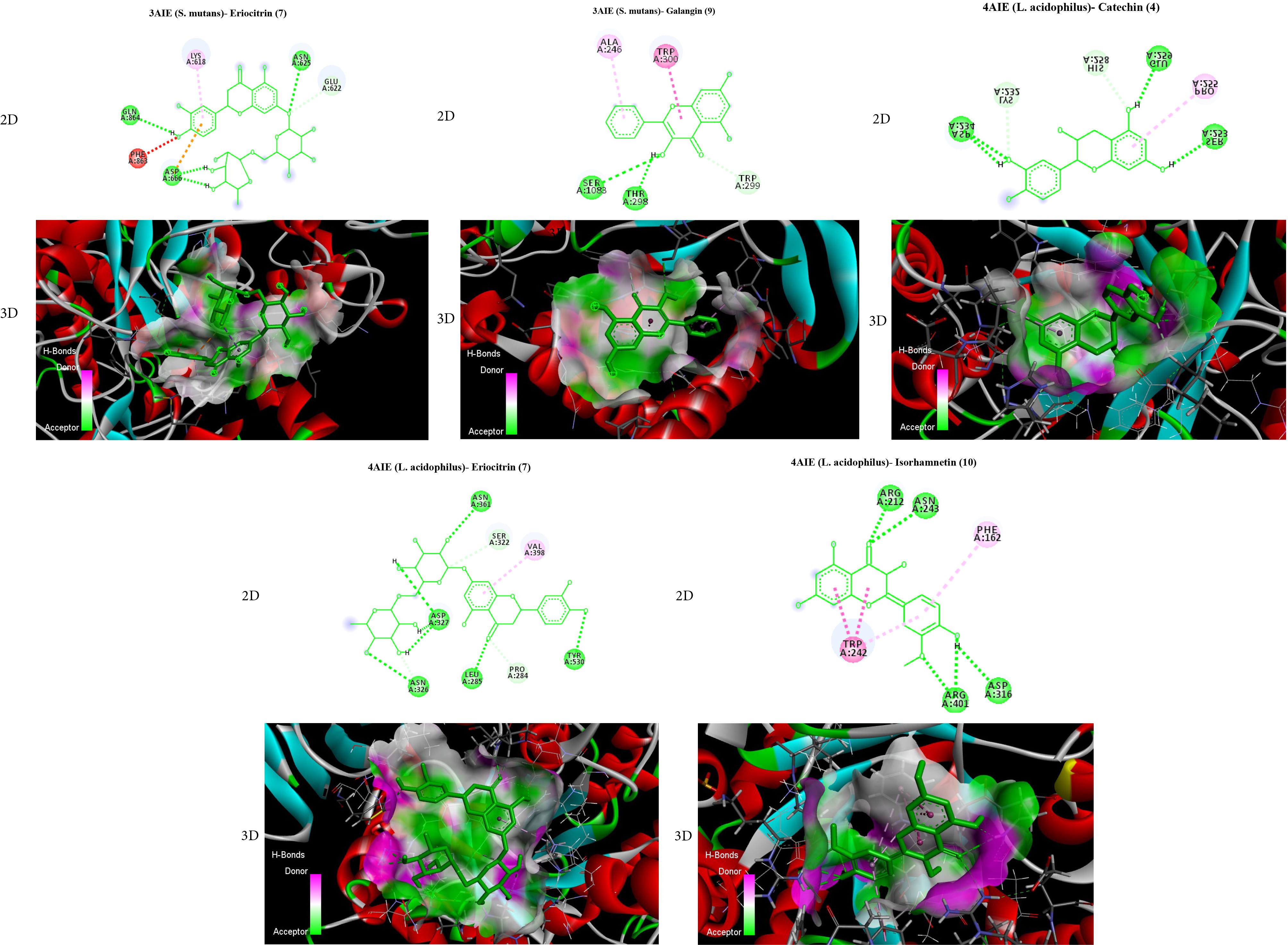
Figure 2.
AutoDock Vina Results of Catechin, Eriocitrin, and Isorhamnetin in the Binding Site of Glucan-1,6-alpha-glucosidase From L. acidophilus, as Well as Eriocitrin and Galangin in the Binding Site of Glucansucrase From S. mutans. Note. Bonds are shown by means of Discovery Studio software (2D and 3D). 2D: 2-dimensional
.
AutoDock Vina Results of Catechin, Eriocitrin, and Isorhamnetin in the Binding Site of Glucan-1,6-alpha-glucosidase From L. acidophilus, as Well as Eriocitrin and Galangin in the Binding Site of Glucansucrase From S. mutans. Note. Bonds are shown by means of Discovery Studio software (2D and 3D). 2D: 2-dimensional
In Vitro: Herein, we have evaluated and compared the antibacterial activity of flavonoid compounds that performed best in the previous section (In silico). According to Figure 3, which deals with the inhibition zone of compounds against target bacteria, eriocitrin + fluoride 0.2% represented the best performance compared to all compounds. Based on the data in Table 3, it seems that the compound eriocitrin has a wide range of antimicrobial activities. Groups a (Fluoride 0.2% + Eriocitrin against S. mutans) and d (Fluoride 0.2% + Eriocitrin against L. acidophilus) were most effective between all compounds, respectively, (IZ = 26 ± 0.5 mm, MIC = 250 µg/mL, MBC = 500 µg/mL and IZ = 31 ± 0.5 mm, MIC = 125 µg/mL, MBC = 250 µg/mL).
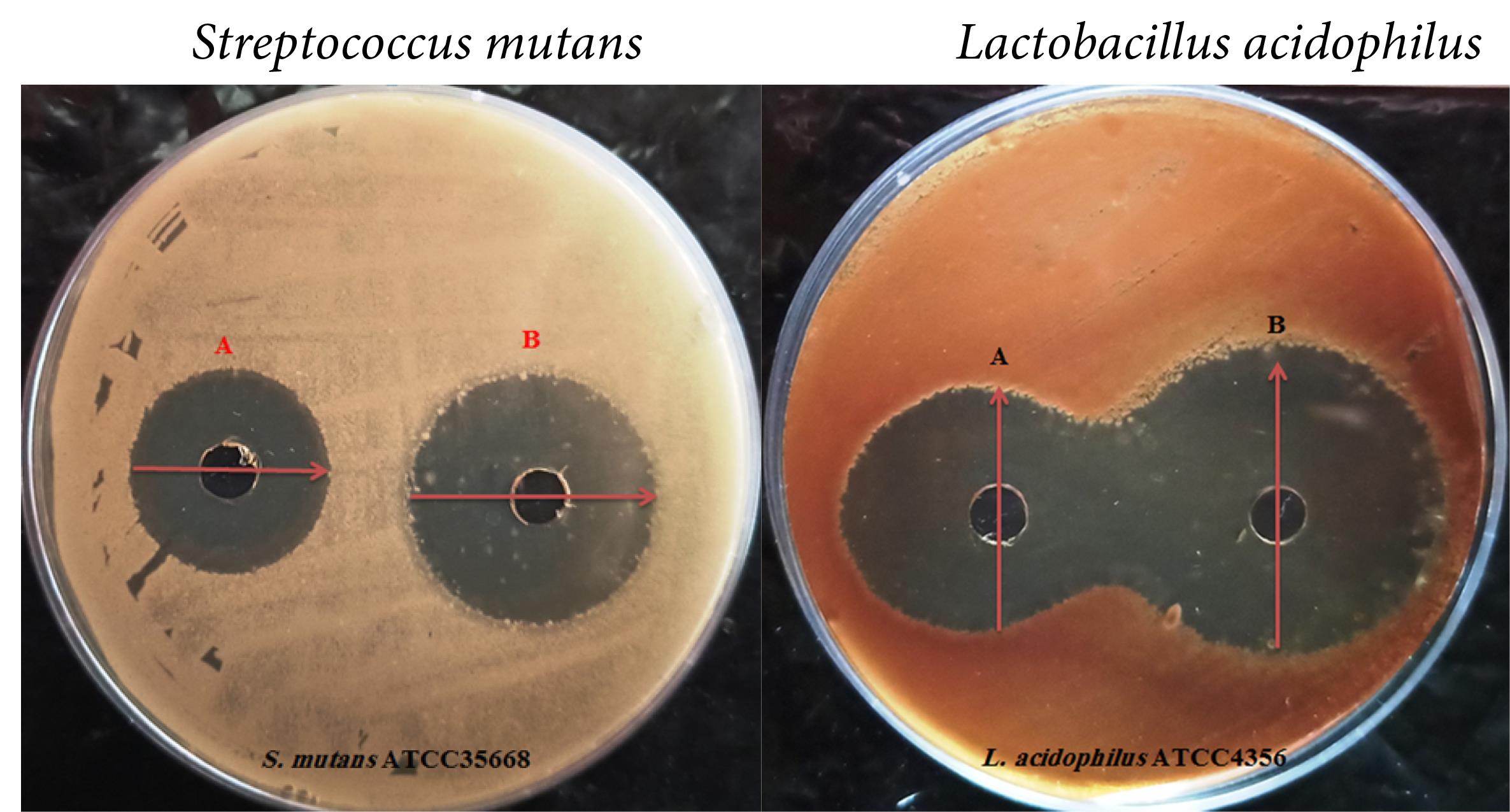
Figure 3.
Antibacterial Results of Flavonoid Compounds: [Streptococcus mutans: A: Control (Fluoride), B: Eriocitrin + Fluoride - Lactobacillus acidophilus: A: Control (Fluoride), B: Eriocitrin + Fluoride]
.
Antibacterial Results of Flavonoid Compounds: [Streptococcus mutans: A: Control (Fluoride), B: Eriocitrin + Fluoride - Lactobacillus acidophilus: A: Control (Fluoride), B: Eriocitrin + Fluoride]
Table 3.
Antibacterial Properties of Flavonoid Compounds
|
Groups
|
|
S.
Mutans
|
L. acidophilus
|
a
(Fluoride + Eriocitrin) |
IZ |
26 ± 0.5 mm |
- |
| MIC |
250 |
- |
| MBC |
500 |
- |
b
(Fluoride + Galangin) |
IZ |
20 ± 0.5 mm |
- |
| MIC |
500 |
- |
| MBC |
≥ 1000 |
- |
c
(Fluoride + Catechin) |
IZ |
- |
25 ± 0.5 mm |
| MIC |
- |
250 |
| MBC |
- |
500 |
d
(Fluoride + Eriocitrin) |
IZ |
- |
31 ± 0.5 mm |
| MIC |
- |
125 |
| MBC |
- |
250 |
e
(Fluoride + Isorhamnetin) |
IZ |
- |
22 ± 0.5 mm |
| MIC |
- |
500 |
| MBC |
- |
≥ 1000 |
| Fluoride (Control) |
IZ |
20 ± 0.5 mm |
27 ± 0.5 mm |
| MIC |
500 |
250 |
| MBC |
≥ 1000 |
500 |
Note. IZ (Millimetre-mm): Inhibition zone; MIC (μg.mL-1): Minimum inhibitory concentration; MBC (μg.mL-1): Minimum bactericidal concentration. (S. mutans wells: Fluoride (Control), Fluoride + Eriocitrin (a), Fluoride + Galangin (b). L. acidophilus wells: Fluoride (Control), Fluoride + Catechin (c), Fluoride + Eriocitrin (d), Fluoride + Isorhamnetin (e)); S. Mutans: Streptococcus mutans; L. acidophilus: Lactobacillus acidophilus.
Discussion
Tooth decay is still considered one of the most serious public health problems, and every year, it imposes a great financial burden on healthcare services all over the world, especially in developing countries (21). One of the most important ways to reduce costs is to prevent rotting. Currently, the most effective way to prevent tooth decay, in addition to reducing the intake of sugary substances, is to use mechanical methods such as brushing and flossing (22). However, other methods and chemical antimicrobial agents such as mouthwashes have been used due to the insufficiency of mechanical methods alone to control plaque and prevent periodontal diseases and tooth decay, on the one hand, and the complex bacterial etiology of periodontal diseases and tooth decay, on the other hand (23). Mouthwashes contain effective preventive substances and play a significant role in improving oral health. From a dental point of view, the ideal mouthwash should have properties such as non-discoloration of teeth and mucous irritation, no toxic effects, and a good taste. However, a mouthwash that has all the mentioned properties has not yet entered the market, and researchers are still attempting to find a mouthwash that has the maximum of these properties (24). Oral streptococci are an important part of the collection of dental plaques, and one of the most important members of this collection is S. mutans, which has been associated with caries in several epidemiological studies and is believed to play the main role in the initiation of caries (25). These bacteria make up to 60% of the natural flora of the surfaces inside the mouth, which can cause the formation of biofilm on the surface of the tooth enamel by producing non-soluble viscous polymer materials such as glucan and levan. The primary mechanism of binding S. mutans is the formation of glucan homopolymers from sucrose by glucosyltransferase. Glucosyltransferase catalyzes two main reactions, which include the breakdown of sucrose into glucose and fructose (sucrazy activity) and the transfer of glucose units at the 6-C-3/C position to produce glucan (transferase activity) (26). The present study was conducted with the aim of investigating the effect of combined mouthwash (Flavonoid compounds and fluoride 0.2%) and fluoride 0.2% on the growth of S. mutans and L. acidophilus. Examining the in silico results showed that most of the flavonoid compounds, which were selected in this research, had the ability to inhibit the bacterial samples in a range of -5.2 to -9.2 kcal/mol. The binding energy determines the degree of binding between the compounds and the active sites of the 3AIE and 4AIE. Whatever this number is more negative, binding between the compound and the 3AIE and 4AIE is stronger. In this research, the flavonoid compound eriocitrin was reported to have an effective and stronger interaction than others. It created the highest hydrogen bonds with amino acids such as asparagine, glutamine, aspartic acid, and glutamic acid by glucansucrase from S. mutans, as well as hydrogen bonds with amino acids such as aspartic acid, asparagine, leucine, tyrosine, proline, and serine by glucan-1, 6-alpha-glucosidase from L. acidophilus. Many studies have been reported on the effects of various flavonoid classes in in silico and in vitro conditions. In this regard, Bao et al sought to identify potential inhibitors targeting S. mutans sortase A. They reported that several similar compounds composed of benzofuran, thiadiazole, and pyrrole, which exhibited good affinities and appropriate pharmacokinetic parameters, were potential inhibitors to impede the catalysis of SrtA (27). Likewise, Babaeekhou and Ghane in silico studied the antimicrobial activity of ginger on cariogenic bacteria and stated that some ginger compounds with high affinity to investigated enzymes can be considered candidate compounds for anti-caries drug development studies (28). Eriocitrin is a flavanone-7-O-glycoside between the flavanone eriodictyol and the disaccharide rutinose. It is commonly found in lemons and other citrus fruits. Similarly, Nisar et al evaluated the pharmacological effects of eriocitrin and concluded that eriocitrin has potent biological actions due to its strong antioxidant, antitumor, anti-allergic, antidiabetic, and anti-inflammatory activities. Eriocitrin is more potent in suppressing oxidative stress in diabetes mellitus and other chronic diseases incurred by excessive oxidative stress. During metabolism, eriocitrin is metabolized by the gut microbiota and a chain of molecules such as eriodictyol, methy-eriodictyol, 3,4-dihydroxyhydrocinnamic acid (DHCA), and much more conjugated molecules (29). In vitro results showed that eriocitrin + fluoride 0.2% has the best performance in comparison to all compounds (IZ = 26 ± 0.5 mm, MIC = 250 µg/mL, MBC = 500 µg/mL against S. mutans and IZ = 31 ± 0.5 mm, MIC = 125 µg/mL, MBC = 250 µg/mL against L. acidophilus). In addition, Miyake and Hiramitsu examined antimicrobial substances against oral bacteria from lemon peel and found that 8-geranyloxypsolaren, 5-geranyloxypsolaren, and 5-geranyloxy-7-methoxycoumarin exhibited high antibacterial activities (30). Moreover, Zajkani et al compared the effect of fluoride 0.2% and a combination mouthwash (xylitol and fluoride) on S. mutans and L. acidophilus growths and reported that the fluoride mouthrinse at different concentrations, because of having a good inhibitory effect on S. mutans and L. acidophilus in both methods, was more effective compared with Fuchs mouthrinse (31). Adamczak et al investigated the antibacterial activities of some flavonoids and organic acids, as well as 13 common flavonoids (flavones, flavonols, and flavanones) and 6 organic acids (aliphatic and aromatic acids). They reported that all tested compounds showed antimicrobial properties, but their biological activity was moderate or relatively low. Bacterial growth was most strongly inhibited by salicylic acid (MIC = 250–500 μg/mL). These compounds were generally more active against gram-negative bacteria, such as Escherichia coli and Pseudomonas aeruginosa, than gram-positive ones, including Enterococcus faecalis and Staphylococcus aureus (32).
Conclusion
According to the results of this study, eriocitrin can be used as a new mouthwash, along with fluoride 0.2%.
Acknowledgements
We appreciate all the professors who participated voluntarily in this investigation.
Authors’ Contribution
Conceptualization: Yasin SarveAhrabi, Behin Omidi.
Funding acquisition: Yasin SarveAhrabi, Behin Omidi.
Investigation: Yasin SarveAhrabi, Behin Omidi, Sarina Nejati Khoei.
Methodology: Yasin SarveAhrabi, Behin Omidi, Sarina Nejati Khoei.
Resources: Yasin SarveAhrabi, Behin Omidi.
Supervision: Yasin SarveAhrabi, Behin Omidi.
Writing–original draft: Yasin SarveAhrabi.
Writing–review & editing: Yasin SarveAhrabi, Behin Omidi.
Competing Interests
The authors declare that they have no conflict of interests.
Funding
This project was supported by the Islamic Azad University, Central Tehran Branch, Tehran, Iran.
References
- Al-Nasser L, Lamster IB. Prevention and management of periodontal diseases and dental caries in the older adults. Periodontol 2000 2020; 84(1):69-83. doi: 10.1111/prd.12338 [Crossref] [ Google Scholar]
- Wade WG. Resilience of the oral microbiome. Periodontol 2000 2021; 86(1):113-22. doi: 10.1111/prd.12365 [Crossref] [ Google Scholar]
- Nomura R, Matayoshi S, Otsugu M, Kitamura T, Teramoto N, Nakano K. Contribution of severe dental caries induced by Streptococcus mutans to the pathogenicity of infective endocarditis. Infect Immun 2020; 88(7):e00897-19. doi: 10.1128/iai.00897-19 [Crossref] [ Google Scholar]
- Pahumunto N, Piwat S, Chanvitan S, Ongwande W, Uraipan S, Teanpaisan R. Fermented milk containing a potential probiotic Lactobacillus rhamnosus SD11 with maltitol reduces Streptococcus mutans: a double-blind, randomized, controlled study. J Dent Sci 2020; 15(4):403-10. doi: 10.1016/j.jds.2020.03.003 [Crossref] [ Google Scholar]
- Yu J, Yan F, Lu Q, Liu R. Interaction between sorghum procyanidin tetramers and the catalytic region of glucosyltransferases-I from Streptococcus mutans UA159. Food Res Int 2018; 112:152-9. doi: 10.1016/j.foodres.2018.06.027 [Crossref] [ Google Scholar]
- Hoshino T, Fujiwara T. The findings of glucosyltransferase enzymes derived from oral streptococci. Jpn Dent Sci Rev 2022; 58:328-35. doi: 10.1016/j.jdsr.2022.10.003 [Crossref] [ Google Scholar]
- Abo Bakr RA, Tawfick MM, Mostafa ZA, Abdulall AK. Virulence traits-based behavior of Streptococcus mutans bacteria from dental plaque and dental caries conditions. Microb Biosyst 2021; 6(1):75-85. doi: 10.21608/mb.2021.100068.1044 [Crossref] [ Google Scholar]
- Wu J, Fan Y, Wang X, Jiang X, Zou J, Huang R. Effects of the natural compound, oxyresveratrol, on the growth of Streptococcus mutans, and on biofilm formation, acid production, and virulence gene expression. Eur J Oral Sci 2020; 128(1):18-26. doi: 10.1111/eos.12667 [Crossref] [ Google Scholar]
- Schulz-Weidner N, Weigel M, Turujlija F, Komma K, Mengel JP, Schlenz MA, et al. Microbiome analysis of carious lesions in pre-school children with early childhood caries and congenital heart disease. Microorganisms 2021;9(9)1904. 10.3390/microorganisms9091904.
- Asgari I, Soltani S, Sadeghi SM. Effects of iron products on decay, tooth microhardness, and dental discoloration: a systematic review. Arch Pharm Pract 2020; 11(1):60-82. [ Google Scholar]
- Karak P. Biological activities of flavonoids: an overview. Int J Pharm Sci Res 2019; 10(4):1567-74. doi: 10.13040/ijpsr.0975-8232.10(4).1567-74 [Crossref] [ Google Scholar]
- Reddy AV, Moniruzzaman M, Madhavi V, Jaafar J. Recent improvements in the extraction, cleanup and quantification of bioactive flavonoids. Stud Nat Prod Chem 2020; 66:197-223. doi: 10.1016/b978-0-12-817907-9.00008-8 [Crossref] [ Google Scholar]
- Farhadi F, Khameneh B, Iranshahi M, Iranshahy M. Antibacterial activity of flavonoids and their structure-activity relationship: an update review. Phytother Res 2019; 33(1):13-40. doi: 10.1002/ptr.6208 [Crossref] [ Google Scholar]
- Akhtar N, Ayoubi R, Kour V, Gautam U, Mannan AU. Natural products for fungal diseases management and prevention. Nat Prod J 2022; 12(2):60-9. doi: 10.2174/2210315511666210512035847 [Crossref] [ Google Scholar]
- Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients 2020; 12(2):457. doi: 10.3390/nu12020457 [Crossref] [ Google Scholar]
- Agati G, Brunetti C, Fini A, Gori A, Guidi L, Landi M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants (Basel) 2020; 9(11):1098. doi: 10.3390/antiox9111098 [Crossref] [ Google Scholar]
- Zahmatkesh K, Akbari Dilmaghani K, Sarveahrabi Y. Synthesis, antimicrobial and molecular docking studies of some new derivatives of 2,3-dihydroquinazolin-4(1H)-one. Acta Chim Slov 2022; 69(3):619-28. doi: 10.17344/acsi.2022.7512 [Crossref] [ Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 2009; 30(16):2785-91. doi: 10.1002/jcc.21256 [Crossref] [ Google Scholar]
- Ghameshlouei S, Zarrabi Ahrabi N, Souldozi A, Sarve Ahrabi Y. Evaluation of the antibacterial and investigation of the molecular docking of new derivatives of 1, 3, 4-oxadiazole as inhibitors of quorum sensing system in the human pathogen Pseudomonas aeruginosa. Avicenna J Clin Microbiol Infect 2021; 8(1):27-33. doi: 10.34172/ajcmi.2021.06 [Crossref] [ Google Scholar]
- Omidi B, Sarve Ahrabi Y. In vitro and in silico evaluation of biological properties of some 1, 3, 4-oxadiazole derivatives against Streptococcus mutans and their interaction with Gbp-C by molecular docking. Avicenna J Dent Res 2021; 13(4):142-7. doi: 10.34172/ajdr.2021.27 [Crossref] [ Google Scholar]
- Clemens J, Gold J, Chaffin J. Effect and acceptance of silver diamine fluoride treatment on dental caries in primary teeth. J Public Health Dent 2018; 78(1):63-8. doi: 10.1111/jphd.12241 [Crossref] [ Google Scholar]
- Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR. Oral diseases: a global public health challenge. Lancet 2019; 394(10194):249-60. doi: 10.1016/s0140-6736(19)31146-8 [Crossref] [ Google Scholar]
- US Department of Health and Human Services Federal Panel on Community Water Fluoridation. US Public Health Service recommendation for fluoride concentration in drinking water for the prevention of dental caries. Public Health Rep. 2015;130(4):318-31. 10.1177/003335491513000408.
- Motallaei MN, Yazdanian M, Tebyanian H, Tahmasebi E, Alam M, Abbasi K. The current strategies in controlling oral diseases by herbal and chemical materials. Evid Based Complement Alternat Med 2021; 2021:3423001. doi: 10.1155/2021/3423001 [Crossref] [ Google Scholar]
- Klein MI, Hwang G, Santos PH, Campanella OH, Koo H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol 2015; 5:10. doi: 10.3389/fcimb.2015.00010 [Crossref] [ Google Scholar]
- Lemos JA, Palmer SR, Zeng L, Wen ZT, Kajfasz JK, Freires IA, et al. The biology of Streptococcus mutans. Microbiol Spectr 2019;7(1):10.1128/microbiolspec.GPP3-0051-2018. 10.1128/microbiolspec.GPP3-0051-2018.
- Luo H, Liang DF, Bao MY, Sun R, Li YY, Li JZ. In silico identification of potential inhibitors targeting Streptococcus mutans sortase A. Int J Oral Sci 2017; 9(1):53-62. doi: 10.1038/ijos.2016.58 [Crossref] [ Google Scholar]
- Babaeekhou L, Ghane M. Antimicrobial activity of ginger on cariogenic bacteria: molecular networking and molecular docking analyses. J Biomol Struct Dyn 2021; 39(6):2164-75. doi: 10.1080/07391102.2020.1745283 [Crossref] [ Google Scholar]
- Yao L, Liu W, Bashir M, Nisar MF, Wan CC. Eriocitrin: a review of pharmacological effects. Biomed Pharmacother 2022; 154:113563. doi: 10.1016/j.biopha.2022.113563 [Crossref] [ Google Scholar]
- Miyake Y, Hiramitsu M. Isolation and extraction of antimicrobial substances against oral bacteria from lemon peel. J Food Sci Technol 2011; 48(5):635-9. doi: 10.1007/s13197-011-0330-3 [Crossref] [ Google Scholar]
- Zajkani E, Zeighami H, Zaeefjou A. Comparision of the effect of fluoride 0.2% and a combination mouthwash (xylitol and fluoride) on Streptococcus mutans and Lactobacillus acidophilus growths. J Dent Med 2017;30(1):57-64. [Persian].
- Adamczak A, Ożarowski M, Karpiński TM. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J Clin Med 2019; 9(1):109. doi: 10.3390/jcm9010109 [Crossref] [ Google Scholar]