Avicenna J Dent Res. 16(1):16-21.
doi: 10.34172/ajdr.1645
Original Article
Effects of Combinations of Hydrogen Peroxide and Vinegar in Different Ratios on the Surface Roughness of Heat-Cure Denture Base Acrylic Resin
Farnaz Firouz 1, 2  , Saeed Nikanjam 2, *
, Saeed Nikanjam 2, *  , Armaghan Shahbazi 1
, Armaghan Shahbazi 1  , Zahra Cheraghi 3
, Zahra Cheraghi 3  , Abdollah Nazari doost 4
, Abdollah Nazari doost 4 
Author information:
1Dental Implants Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Prosthodontics, Faculty of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
3Department of Biostatistics, Faculty of Health, Hamadan University of Medical Sciences, Hamadan, Iran
4Undergraduate Student, Faculty of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Background: Complete dentures are at high risk of contamination, and their disinfection is imperative to prevent cross-contamination. Also, chemical disinfecting agents can effectively eliminate microorganisms. This study aimed to assess the effect of combinations of hydrogen peroxide (HP) and vinegar in different ratios on the surface roughness of heat-cure denture base acrylic resin.
Methods: This in vitro, experimental study evaluated 40 heat-cure acrylic resin specimens that were flasked and heated at 70 °C for 9 hours for heat polymerization. The acrylic specimens were cut into small cubic pieces measuring 20×20×3 mm using a cutting machine and polished with metallographic abrasive paper. The specimens were randomized into 4 groups of control (artificial saliva) and HP and vinegar in 1:1, 1:3, and 3:1 ratios (three experimental groups). Then, they were immersed in the respective solutions for 8 hours/day for one month. Their surface roughness (Ra) was measured before and after immersion by a profilometer. The data were analyzed using a one-way analysis of variance (α=0.05).
Results: The immersion of acrylic specimens in HP+vinegar in 1:1 and 1:3 ratios did not cause a significant change in their surface roughness (P>0.05). However, the surface roughness significantly decreased after immersion in HP+vinegar in a 3:1 ratio (P=0.032). Despite the reduction in the surface roughness of specimens in the 3:1 group, the difference in surface roughness was not significant among the four groups after immersion (P>0.05).
Conclusion: Combinations of HP and vinegar in different ratios appear to be suitable for cleaning removable dentures due to their insignificant effects on the surface roughness of acrylic resin.
Keywords: Hydrogen peroxide, Acetic acid, Roughness, Acrylic resins, Polymerization
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Firouz F, Nikanjam S, Shahbazi A, Cheraghi Z, Nazari doost A. Effects of combinations of hydrogen peroxide and vinegar in different ratios on the surface roughness of heat-cure denture base acrylic resin. Avicenna J Dent Res. 2024; 16(1):16-21. doi:10.34172/ajdr.1645
Background
Optimal cleaning and disinfection of complete and partial dentures play a fundamental role in the oral mucosal health of denture wearers (1). However, it is often a difficult task for the elderly due to the presence of underlying systemic conditions, dementia, and compromised hand skills or movement coordination. Irrespective of adverse esthetic effects, poor oral hygiene leads to the formation of biofilm and the subsequent development of oral mucosal inflammation and opportunistic infections (2).
Physical and mechanical changes in denture base acrylic resins due to the effects of oral environmental conditions or cleansing agents are a common concern for many dental clinicians in the fabrication of removable partial or complete dentures. However, hygienic measures are also an integral part of oral health and are imperative for denture wearers (3). Removable partial or complete dentures are at high risk of contamination, and their disinfection is imperative to prevent cross-contamination. Different microorganisms with various levels of pathogenicity have been isolated from dental prostheses, which are capable of causing pneumonia, conjunctivitis, and meningitis (4,5). According to the infection control protocol of the American Dental Association, dental prostheses should be disinfected prior to transfer to a laboratory and delivery to patients (3).
Evidence shows that chemical disinfecting agents can effectively eliminate microorganisms and microbial plaque (5). Physical and mechanical methods are not as effective as chemical cleansing agents in the reduction of denture microorganisms (6,7). However, chemical agents often change the physical and mechanical properties of acrylic resins and lead to alterations in their color, flexural strength, and surface roughness. Increased surface roughness over time enhances microbial adhesion and the accumulation of food particles on the acrylic surface of a denture (8,9), eventually resulting in oral mucosal irritation and inflammation (10). Chemical disinfecting agents are among the main causes of increased denture surface roughness (11). The optimal efficacy of chemical denture cleansing agents in the dissolution and elimination of food particles, biofilm, and stains caused by cigarette smoking has been documented in previous studies (12,13). However, they have drawbacks, such as causing whitish discoloration and roughening the denture surface. Bleaching products have a bad odor, cause discoloration, and degrade the denture soft liners. Weak acids such as citric acid or household vinegar are commonly used for the elimination of water scales. They attack the inorganic phosphate parts of the scales and decrease scale buildup. Vinegar can also eliminate microorganisms, but it is less effective than the bleaching agents (14). The immersion of dentures in vinegar or hydrogen peroxide (HP) alone does not seem to effectively eliminate microorganisms responsible for denture stomatitis. A combination of vinegar and HP, however, can noticeably eliminate Candida albicans and Staphylococcus aureus (15). It has been reported that a combination of HP and vinegar can also decrease microbial plaques on the denture surface. However, vinegar may roughen the denture surface since it is acidic. This topic needs further investigation since increased surface roughness enhances microbial plaque accumulation.
The efficacy of sodium hypochlorite and vinegar for denture surface disinfection has been previously investigated. However, information regarding the effect of a combination of HP and vinegar on the surface roughness of dentures is lacking. Thus, this study sought to assess the effects of combinations of HP and vinegar in different ratios on denture surface roughness.
Materials and Methods
This in vitro experimental study examined 40 heat-cure acrylic resin specimens. The sample size was calculated to be 10 in each group according to a study by Nematollahi et al (16), assuming a 95% confidence interval, alpha = 0.05, beta = 0.2, and 90% study power.
For the fabrication of acrylic specimens, white gypsum (Pars Dandan, Tehran, Iran) was poured into the lower compartment of the flask, and a glass slab was placed over it. After setting the gypsum and applying biofilm (spacer), metal molds with a diameter of 17 mm and a height of 6 mm were glued to the glass slab. Another glass slab was placed over the molds, and mold stone was poured into the upper compartment (type III mold stone; Pars Dandan, Tehran) such that the metal molds were embedded in stone. After setting the mold stone, the third-phase stone was added with the glass slab in place, and the flask was closed and subjected to pressure for the stone to set (11). Acrylic resin (Bayer, Germany) was applied after the completion of flasking, wax burnout, and irrigation. After the final packing of the flask, it was heated in a furnace (Type 556, Kavo EVL, Germany) and underwent relatively slow polymerization at 70°C for 9 hours. The specimens were immersed in distilled water at 37 ± 1 °C for 50 ± 2 minutes to release the free monomers. Next, they were cut into six equal pieces measuring 20 × 20 mm with a 3 mm thickness with a cutting machine and a diamond disc under air and water coolant such that the temperature did not exceed 30 °C. The specimens were then finished and polished with metallographic abrasive paper with approximately 30 µm (p 500), 18 µm (p 1000), and 15 µm (p 1200) abrasiveness. All specimens were fabricated at a temperature of 23 ± 2 °C and a relative humidity of 50 ± 10% (16). To achieve the desired dimensions, the specimens underwent standard polishing, and 40 acrylic resin specimens fabricated as such were randomized into one control (artificial saliva) and three experimental groups for immersion in a combination of HP and vinegar in 1:1, 1:3, and 3:1 ratios, respectively. The specimens were immersed in 100 cc of the respective solution for 8 hours/day. They were then removed from the disinfecting agent, rinsed, and immersed in distilled water for 16 hours. The solutions were refreshed daily, and this process was repeated for one month.
The surface roughness of the specimens was measured at baseline (before immersion) and after immersion by using a profilometer (Surface Roughness Tester, TR-200 Plus, USA). The measurements were made at three points for each specimen, and the mean of the three Ra (mean surface roughness) values was calculated and reported as the mean surface roughness of the respective specimen (16).
Finally, two specimens were randomly selected from each group and underwent scanning electron microscopy (SEM). For this purpose, first, they were cleaned in an ultrasonic bath for 5 minutes, adhered to an aluminum stub by a conductive tape, and sputter coated (JFC-1100E ION SPUTTER, JEOL, Japan) with gold-palladium alloy for 10 minutes. Then, they were inspected under a SEM (JEOL JSM-840A, JEOL Ltd., Tokyo, Japan) and photographed.
The Shapiro-Wilk test was used to assess the normality of data distribution, which showed the normal distribution of data in two out of four groups. Thus, to increase the statistical power, the mean surface roughness (Ra value) of the groups was compared using analysis of variance (ANOVA). STATA 11 software was utilized for all statistical analyses at a 0.05 level of significance.
Results
Table 1 presents the mean surface roughness of the four groups at baseline and after immersion. Within-group comparison of surface roughness indicated no significant change in surface roughness after immersion in any group, except for the HP + vinegar group in a 3:1 ratio, which demonstrated a significant reduction in surface roughness after immersion (P= 0.032). The ANOVA results revealed no significant difference in the surface roughness of the groups after immersion (P = 0.882). In addition, Table 2 provides a pairwise comparison between the mean differences (before and after immersion) of different groups.
Table 1.
Mean Surface Roughness of the Four Groups at Baseline and After Immersion (n = 10)
|
Group
|
Mean±SD of Surface Roughness Before Immersion
|
Mean±SD of Surface Roughness After Immersion
|
Mean Difference
|
P
Value
|
| HP + vinegar in 1:3 ratio |
0.41 ± 0.40 |
0.42 ± 0.45 |
0.01 ± 0.14 |
0.436 |
| HP + vinegar in 3:1 ratio |
0.32 ± 0.26 |
0.28 ± 0.26 |
-0.04 ± 0.05 |
0.032 |
| Vinegar + HP in 1:1 ratio |
0.35 ± 0.24 |
0.36 ± 0.39 |
0.01 ± 0.39 |
0.453 |
| Control group |
0.30 ± 0.12 |
0.30 ± 0.12 |
0.00 ± 0.00 |
0.566 |
Note. HP: Hydrogen peroxide; SD: Standard deviation.
Table 2.
Pairwise Comparison Between Mean Differences (Before and After Immersion) of Different Groups
|
Compared Groups
|
P
Value
|
| HP + vinegar in a 1:3 ratio with control group |
0.124 |
| HP + vinegar in a 3:1 ratio with control group |
0.098 |
| HP + vinegar in a1:1 ratio with control group |
0.12 |
| HP + vinegar in a 1:3 ratio with HP + vinegar in a 3:1 ratio |
0.045 |
| HP + vinegar in a 1:3 ratio with HP + vinegar in a 1:1 ratio |
0.230 |
| HP + vinegar in a 3:1 ratio with HP + vinegar in a 1:1 ratio |
0.033 |
Note. HP: Hydrogen peroxide.
Figures 1–4 show the SEM micrographs of specimens in the four groups. The minimum surface roughness was noted in the HP + vinegar group in a 3:1 ratio, followed by the control group. The maximum surface roughness was found in the HP + vinegar group in a 1:3 ratio.
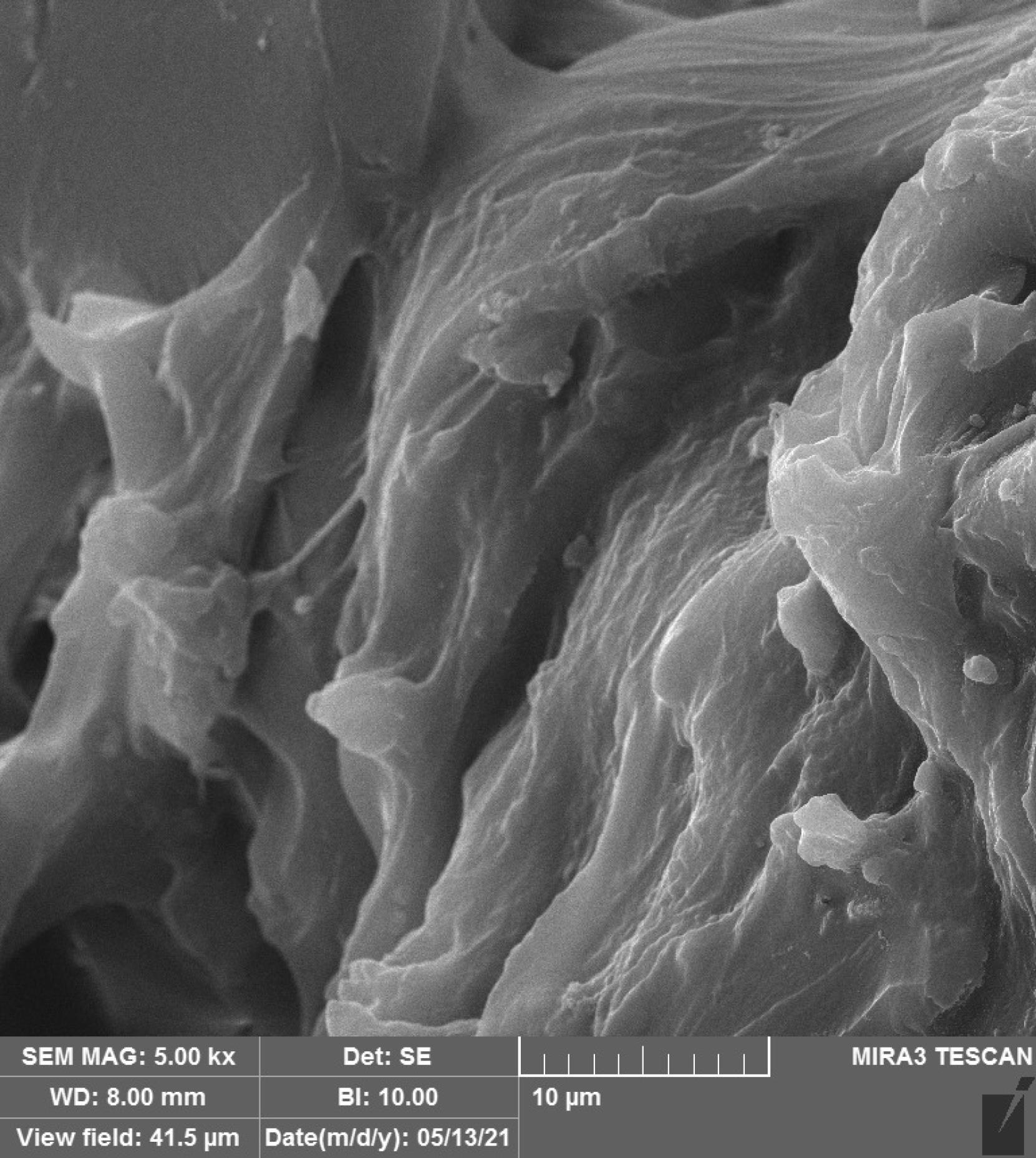
Figure 1.
SEM Micrograph of the Surface Roughness of a Specimen in HP + Vinegar Group in a 1:1 Ratio. Note. HP: Hydrogen peroxide; SEM: Scanning electron microscopy
.
SEM Micrograph of the Surface Roughness of a Specimen in HP + Vinegar Group in a 1:1 Ratio. Note. HP: Hydrogen peroxide; SEM: Scanning electron microscopy
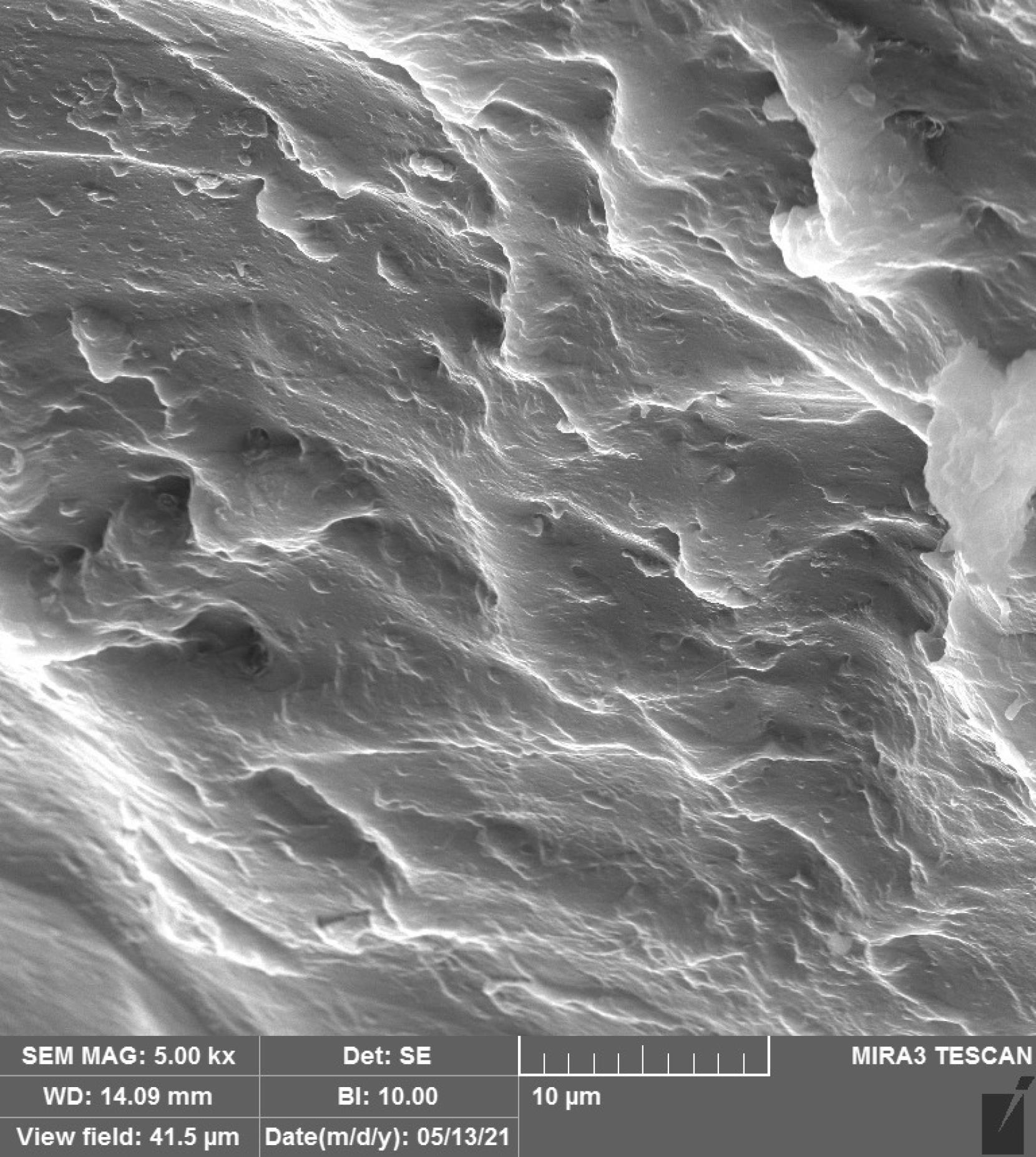
Figure 2.
SEM Micrograph of the Surface Roughness of a Specimen in HP + Vinegar Group in a 3:1 Ratio. Note. HP: Hydrogen peroxide; SEM: Scanning electron microscopy
.
SEM Micrograph of the Surface Roughness of a Specimen in HP + Vinegar Group in a 3:1 Ratio. Note. HP: Hydrogen peroxide; SEM: Scanning electron microscopy
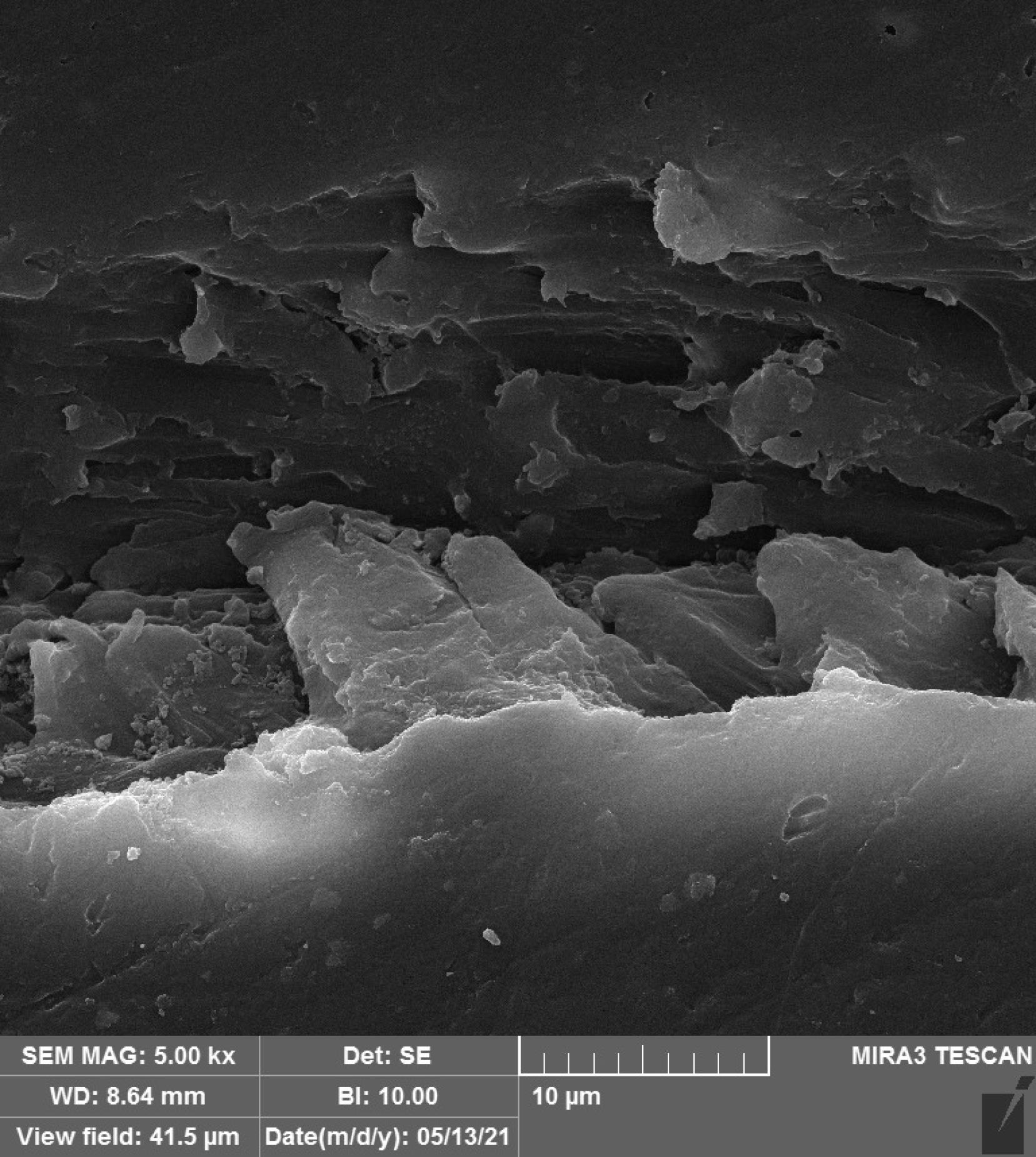
Figure 3.
SEM Micrograph of the Surface Roughness of a Specimen in HP + Vinegar Group in a 1:3 Ratio. Note. HP: Hydrogen peroxide; SEM: Scanning electron microscopy
.
SEM Micrograph of the Surface Roughness of a Specimen in HP + Vinegar Group in a 1:3 Ratio. Note. HP: Hydrogen peroxide; SEM: Scanning electron microscopy
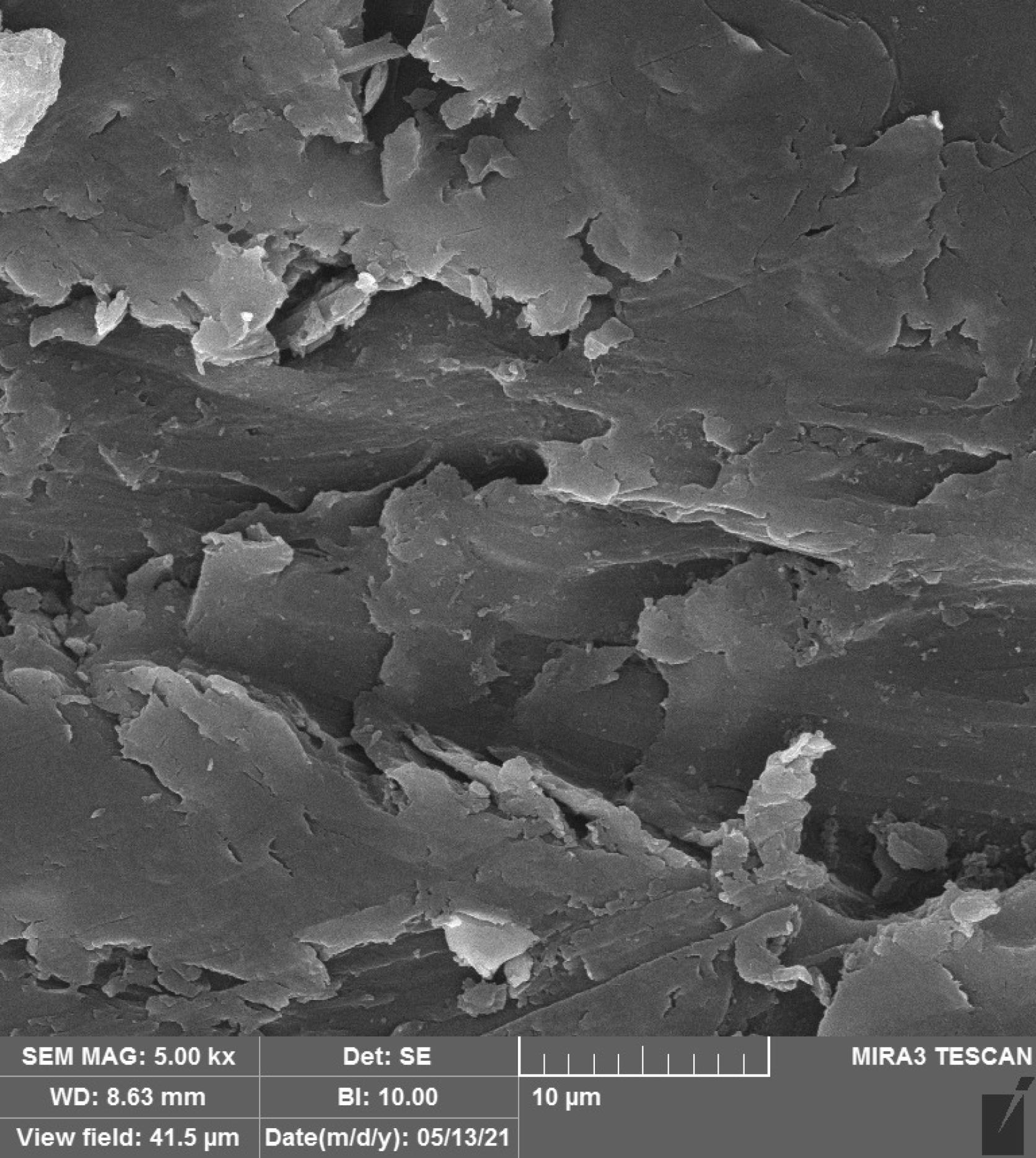
Figure 4.
SEM Micrograph of the Surface Roughness of a Control Specimen. Note. SEM: Scanning electron microscopy
.
SEM Micrograph of the Surface Roughness of a Control Specimen. Note. SEM: Scanning electron microscopy
Discussion
Denture cleansing and disinfecting agents can adversely affect the surface characteristics of dentures and may increase their surface roughness, which would enhance plaque accumulation. This study assessed the effect of combinations of HP and vinegar in different ratios on denture surface roughness. The results demonstrated that the immersion of acrylic specimens in the HP + vinegar solution in 1:1 and 1:3 ratios did not cause a significant change in their surface roughness (P > 0.05). However, the surface roughness significantly decreased after immersion in HP + vinegar in a 3:1 ratio (P= 0.032). Despite the reduction in the surface roughness of specimens in the 3:1 group, the difference in surface roughness was not significant among the four groups after immersion (P> 0.05).
Boonsoe et al (17) found that 5% vinegar changed the surface roughness of acrylic resins after 2 and 3 months, which contradicts our results, which may be due to the difference in assessment time points (1 month in the present study vs. 2–3 months in the mentioned study) and different heat curing protocols of specimens in their study (90 minutes at 74 °C and 30 minutes at 100 °C). Other differences included the different immersion times of specimens for monomer release (24 hours in their study vs. 50 minutes in our study) and the use of vinegar alone in their study versus vinegar and HP in the present study. Nematollahi et al (16) concluded that 2.5% white vinegar increased the surface roughness (both Ra and Rz values) of Bayer and Acropars acrylic resins. They indicated that vinegar caused deep grooves on the acrylic surface, especially in Acropars acrylic resin. The differences between their results and ours may be because they used vinegar alone, while we utilized a combination of vinegar and HP. Pereira et al (18) evaluated the effect of vinegar on the microhardness and surface roughness (Ra) of Meliodent acrylic resin. They reported no significant effect of vinegar on these parameters, which is in agreement with the present results despite different heat curing conditions and larger size of specimens in their study. As mentioned earlier, Nematollahi et al (16) and Boonsoe et al (17) used vinegar alone, which increased the surface roughness of acrylic resins, while we employed a combination of vinegar and HP. The results of the present study also indicated that increasing the amount of vinegar and decreasing the amount of HP could increase the surface roughness (Ra), but not significantly. These results confirmed that vinegar increases the surface roughness of the denture base and, therefore, should better be used with other solutions. The aggravation of surface roughness by the effect of vinegar may be due to the high concentration of citric acid in this solution. Citric acid is the main and most important constituent of vinegar, and its acidic property causes the hydrolysis of polymers in the resin surface and the subsequent release of monomers. Thus, it has a corroding effect on the acrylic surface and causes the chemical hydrolysis of the acrylic polymer (19).
The present study evaluated the effect of HP in combination with vinegar on surface roughness, while most previous studies assessed the effect of sodium hypochlorite. Ebadian et al (11) showed that sodium hypochlorite increased the surface roughness of acrylic resins. The increased surface roughness of the denture base was explained to be due to the corroding effect of sodium hypochlorite. However, glutaraldehyde had no significant impact on surface roughness after 7 days. Firouz et al (20) reported similar results and concluded that, unlike glutaraldehyde, sodium hypochlorite increased acrylic surface roughness, and this increase was greater than that caused by vinegar. Aziz et al (21) demonstrated that sodium hypochlorite caused greater roughening of the acrylic surface compared with vinegar. In fact, disinfecting agents at high concentrations cause the degradation of the acrylic matrix and the release of free monomers, leading to an eventual increase in surface roughness. Thus, it is recommended that solutions with lower corroding effects and lower dissolution be used, or it is suggested that they be used in combination with other disinfecting agents (20). Due to the high corrosion caused by sodium hypochlorite, HP was utilized in combination with vinegar in the present study, and the results revealed that this combination had no adverse effect on the surface roughness of Bayer acrylic resin, irrespective of the mixing ratio. The reason is probably the lower corroding effect of HP compared with sodium hypochlorite (22,23). Pereira et al (18) concluded that HP had no significant impact on the physical and mechanical properties of acrylic resin, such as microhardness and roughness (Ra), in the short-term (150 hours) and long-term (300 hours) periods. Their results are in line with those of the present study, despite the fact that they only used HP.
The antimicrobial activity of vinegar in combination with HP was not evaluated in the present study. However, many previous studies have confirmed the antimicrobial activity of vinegar (24,25), HP, and sodium hypochlorite (23). However, HP is preferred to sodium hypochlorite due to the high corrosive properties of sodium hypochlorite and its adverse effects on the physical and mechanical properties of acrylic resin (16). Thus, by using a combination of HP and vinegar, we can probably benefit from the antimicrobial properties of the mixture with no or minimal adverse effects on acrylic surface roughness. However, studies on the antimicrobial properties of this mixture against different microorganisms are required to make a final judgment in this respect. Soto et al (26) indicated that the combinations of HP and vinegar in 1:1, 1:3, and 3:1 ratios eliminated the Candida albicans biofilm. However, they did not assess the effect of these combinations on the physical and mechanical properties of the heat-cure acrylic resin, and to the best of our knowledge, the present study appears to be the first to address this topic. Acetic acid in vinegar has a synergistic effect with HP and reacts with it to form peracetic acid, with increased antimicrobial activity. Therefore, it can be utilized at lower concentrations to ensure no or minimal adverse effects on the mechanical properties of the acrylic resin (26). It should also be noted that HP has antiviral effects on severe acute respiratory syndrome coronavirus 2 as well, which is the culprit responsible for coronavirus disease 19 (27). Vinegar also has strong antiviral effects, especially against the severe acute respiratory syndrome coronavirus 2 (28). Hence, considering the present results, combinations of HP and vinegar may be suitable for cleansing complete or partial dentures, especially during the coronavirus disease 19 pandemic.
Heat-cure acrylic resin was used in the present study due to its higher resistance compared with nylon and thermoplastic acrylic resins, as well as its popularity. It is hydrophilic. However, its water sorption is lower than that of other resins. This type of resin has a higher molecular weight, higher cross-linking, and a crystalline structure, which results in higher strength and hardness and higher resistance to dissolution (19). Nematollahi et al (16) discussed that the effect of disinfecting agents on surface roughness (Ra and Rz) depends on the type of acrylic resin as well. They demonstrated that Acropars acrylic resin was more affected by acidic disinfecting agents such as vinegar compared with Bayer acrylic resin. Further, Bayer was more significantly affected by sodium hypochlorite. Thus, Bayer (Melodent, Germany) was utilized in this study, which is highly popular worldwide. Moreover, different types of vinegar have been used in the literature. Vinegar is available in two commercial and household forms. Furthermore, different vinegars are made of a variety of fruits. Thus, the type of vinegar may also affect the results (19). The concentration of vinegar, technique of acrylic resin polymerization, thickness and dimensions of the specimens, polishing technique, immersion time, and duration of immersion can all affect the results. In the present study, the specimens were immersed in the respective solutions for 6 hours/daily for one month (16,20).
This study had an in vitro design. Thus, the results must be generalized with caution to the clinical setting. Further studies over longer periods of time are required to assess the effects of different combinations of HP and vinegar on the other properties of various types of denture base acrylic resins as well as clinical isolates and bacterial biofilm.
Conclusion
Combinations of HP and vinegar in different ratios appear to be suitable for cleansing removable dentures due to their insignificant effects on the surface roughness of Bayer acrylic resin.
Authors’ Contribution
Conceptualization: Farnaz Firouz
Data curation: Abdollah Nazari doost.
Formal analysis: Zahra Cheraghi.
Funding acquisition: Farnaz Firouz.
Investigation: Saeed Nikanjam.
Methodology: Farnaz Firouz, Saeed Nikanjam.
Project administration: Armaghan Shahbazi.
Resources: Abdollah Nazari doost.
Software: Saeed Nikanjam.
Supervision: Farnaz Firouz, Saeed Nikanjam.
Validation: Farnaz Firouz, Saeed Nikanjam.
Visualization: Abdollah Nazari doost, Zahra Cheraghi.
Writing–original draft: Farnaz Firouz, Saeed Nikanjam.
Writing–review & editing: Farnaz Firouz, Saeed Nikanjam.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The study protocol was approved by the Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1399.138).
Funding
The study was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 9903271841).
References
- Shay K. Denture hygiene: a review and update. J Contemp Dent Pract 2000; 1(2):28-41. [ Google Scholar]
- Kulak-Ozkan Y, Kazazoglu E, Arikan A. Oral hygiene habits, denture cleanliness, presence of yeasts and stomatitis in elderly people. J Oral Rehabil 2002; 29(3):300-4. doi: 10.1046/j.1365-2842.2002.00816.x [Crossref] [ Google Scholar]
- Infection control recommendations for the dental office and the dental laboratory. ADA Council on Scientific Affairs and ADA Council on Dental Practice. J Am Dent Assoc. 1996;127(5):672-80. 10.14219/jada.archive.1996.0280.
- Powell GL, Runnells RD, Saxon BA, Whisenant BK. The presence and identification of organisms transmitted to dental laboratories. J Prosthet Dent 1990; 64(2):235-7. doi: 10.1016/0022-3913(90)90185-f [Crossref] [ Google Scholar]
- Sharafeddin F, Sadeghi A, Kohanteb G. Comparison of the effect of deconex (solarsept), micro 10 and Cidex in disinfecting dental instruments. J Dent 2005; 6(1-2):38-46. [ Google Scholar]
- Kulak Y, Arikan A, Kazazoglu E. Existence of Candida albicans and microorganisms in denture stomatitis patients. J Oral Rehabil 1997; 24(10):788-90. doi: 10.1046/j.1365-2842.1997.00550.x [Crossref] [ Google Scholar]
- Webb BC, Thomas CJ, Harty DW, Willcox MD. Effectiveness of two methods of denture sterilization. J Oral Rehabil 1998; 25(6):416-23. doi: 10.1046/j.1365-2842.1998.00266.x [Crossref] [ Google Scholar]
- Radford DR, Challacombe SJ, Walter JD. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit Rev Oral Biol Med 1999; 10(1):99-116. doi: 10.1177/10454411990100010501 [Crossref] [ Google Scholar]
- Bulad K, Taylor RL, Verran J, McCord JF. Colonization and penetration of denture soft lining materials by Candida albicans. Dent Mater 2004; 20(2):167-75. doi: 10.1016/s0109-5641(03)00088-5 [Crossref] [ Google Scholar]
- Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater 1997; 13(4):258-69. doi: 10.1016/s0109-5641(97)80038-3 [Crossref] [ Google Scholar]
- Ebadian B, Poorsina F, Saghaei S. Evaluation of disinfecting effect of 0.5% sodium hypochlorite and 2% glutaraldehyde on heat-cure acrylic resin. J Mashhad Dent Sch 2007;31(3):217-22. [Persian].
- Gornitsky M, Paradis II, Landaverde G, Malo AM, Velly AM. A clinical and microbiological evaluation of denture cleansers for geriatric patients in long-term care institutions. J Can Dent Assoc 2002; 68(1):39-45. [ Google Scholar]
- Saraç D, Saraç YS, Kurt M, Yüzbaşioğlu E. The effectiveness of denture cleansers on soft denture liners colored by food colorant solutions. J Prosthodont 2007; 16(3):185-91. doi: 10.1111/j.1532-849X.2006.00170.x [Crossref] [ Google Scholar]
- Moore TC, Smith DE, Kenny GE. Sanitization of dentures by several denture hygiene methods. J Prosthet Dent 1984; 52(2):158-63. doi: 10.1016/0022-3913(84)90087-8 [Crossref] [ Google Scholar]
- Pavarina AC, Pizzolitto AC, Machado AL, Vergani CE, Giampaolo ET. An infection control protocol: effectiveness of immersion solutions to reduce the microbial growth on dental prostheses. J Oral Rehabil 2003; 30(5):532-6. doi: 10.1046/j.1365-2842.2003.01093.x [Crossref] [ Google Scholar]
- Nematollahi F, Saghiri M, Mobayeni M, Momeni Moghaddam M. Evaluating the effect of four chemical disinfectants on surface roughness of acrylic resin denture base material (in vitro evaluation). J Res Dent Sci 2014; 11(3):160-6. [ Google Scholar]
- Boonsoe N, Kanson R, Sookto T. Effect of denture cleansers on physical and mechanical properties of denture base acrylic resin. Int Dent Med J Adv Res 2019; 5:1-5. [ Google Scholar]
- Pereira CJ, Genari B, Leitune VC, Collares FM, Samuel SM. Effect of immersion in various disinfectant solutions on the properties of a heat-cured acrylic resin. Rev Gaucha Odontol 2019; 67:e20190052. doi: 10.1590/1981-86372019000523090 [Crossref] [ Google Scholar]
- Kodir K, Tanti I, Odang RW. Surface roughness of denture bases after immersion in fishcake vinegar solution. J Phys Conf Ser 2017; 884(1):012075. doi: 10.1088/1742-6596/884/1/012075 [Crossref] [ Google Scholar]
- Firouz F, Izadi A, Khalesi M, Vafaei F, Beikmohammadi S, Heidari B. Assessment of effect of chemical disinfectants on surface roughness of heat-polymerized denture base acrylic resin. Avicenna J Clin Med 2012;19(3):57-60. [Persian].
- Aziz S, Malik M, Nazir S, Mir S. Assessment of impact of denture cleansers on surface roughness of heat cure acrylic dentures. J Adv Med Dent Sci Res 2020; 8(9):98-100. doi: 10.21276/jamdsr [Crossref] [ Google Scholar]
- Rutala WA, Weber DJ. Disinfection of endoscopes: review of new chemical sterilants used for high-level disinfection. Infect Control Hosp Epidemiol 1999; 20(1):69-76. doi: 10.1086/501544 [Crossref] [ Google Scholar]
- Lineback CB, Nkemngong CA, Wu ST, Li X, Teska PJ, Oliver HF. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob Resist Infect Control 2018; 7:154. doi: 10.1186/s13756-018-0447-5 [Crossref] [ Google Scholar]
- Jafari AA, Falah-Tafti A, Lotfi-Kamran MH, Zahraeii Z, Kazemi A. Vinegar as a removing agent of Candida albicans from acrylic resin plates. Jundishapur J Microbiol 2012; 5(2):388-92. doi: 10.5812/jjm.2499 [Crossref] [ Google Scholar]
- Basson NJ, Quick AN, Thomas CJ. Household products as sanitising agents in denture cleansing. J Dent Assoc S Afr 1992; 47(10):437-9. [ Google Scholar]
- Soto AF, Mendes EM, Arthur RA, de Cássia Negrini T, Lamers ML, Mengatto CM. Antimicrobial effect and cytotoxic activity of vinegar-hydrogen peroxide mixture: a possible alternative for denture disinfection. J Prosthet Dent 2019;121(6):966.e1-966.e6. 10.1016/j.prosdent.2019.02.019.
- Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J Prosthodont 2020; 29(7):599-603. doi: 10.1111/jopr.13220 [Crossref] [ Google Scholar]
- Pagani I, Ghezzi S, Clementi M, Poli G, Bussi M, Pianta L, et al. Vinegar and its active component acetic acid inhibit SARS-CoV-2 infection in vitro and ex vivo. bioRxiv [Preprint]. July 20, 2020. Available from: https://www.biorxiv.org/content/10.1101/2020.07.08.193193v2.full.