Avicenna J Dent Res. 16(1):46-56.
doi: 10.34172/ajdr.1607
Review Article
Utilizing Saliva Biomarkers for Diagnostic Purposes
Fatemeh Ahmadi -Motamayel 1  , Ali Mahdavinezhad 2
, Ali Mahdavinezhad 2  , Seyedeh Sareh Hendi 3, *
, Seyedeh Sareh Hendi 3, * 
Author information:
1Dental Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Genetics and Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
3Department of Endodontics, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Saliva, as a complex fluid composed of secretory products from salivary glands, plays different roles in the oral cavity. Saliva has potentially been used for the diagnosis of viral, bacterial, and systemic diseases in the last few years. Individuals with minimal training can collect saliva as a diagnostic fluid easily and non-invasively. Saliva is a simple and economical method for screening the public. However, saliva has various levels of variability and instability. A systematic search of published literature was performed on Medline using PubMed. Several keywords, including saliva, systemic disease, disease, health, and diagnosis, were used in this search. This review discusses the diagnostic potential of salivary systemic diseases such as infectious, malignant, and other systemic diseases.
Keywords: Diagnosis, Saliva, Systemic disease, Oral disease, Cancer
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Ahmadi Motamayel F, Mahdavinezhad A, Hendi SS. Utilizing saliva biomarkers for diagnostic purposes. Avicenna J Dent Res. 2024; 16(1):46-56. doi:10.34172/ajdr.1607
Search Strategy
This review focuses on the diagnostic potential of salivary systemic diseases, including malignant, infectious, and other systemic diseases. Accordingly, a systematic search of published literature was conducted on Medline using Pubmed. Several key words, including saliva, disease, systemic disease, diagnosis, and health, were utilized in this search. More than 200 articles were extracted, and after review by the authors, 102 of them were selected in the field of diagnostic use of saliva, such as metabolic, systemic, endocrine, periodontal, and other diseases. Articles that were in languages other than English and whose scope of study was not diagnostic use of saliva were removed from the investigation.
Literature Review
Saliva, as a complex fluid composed of secretory products from salivary glands,plays a wide range of roles in the oral cavity and is of pivotal importance for maintaining oral health (1,2).
Saliva contains 99% water and 1% electrolytes, mucins, antiseptic substances, immunoglobulin, proteins, and various enzymes (1,3). Some of the main functions of saliva are lubrication, mastication, and taste perception, as well as the prevention of oral infection and dental caries (1).
The non-invasive nature of the test makes saliva an interesting option for epidemiologic studies.
Samples collected from saliva are easy to obtain and reliable for the evaluation of physiological conditions, estimation of blood levels of some drugs and hormones, oral signs of systemic disease diagnosis, and severity of an illness assessment. Nowadays, saliva is increasingly used for diagnosing different diseases. Moreover, the acceptability of saliva for being utilized in diagnosing a disease or monitoring its progress has been considerably raised in recent years (4). Saliva has many metabolites that can be employed for the detection of diseases and the investigation of pathophysiological states; thus, it can be applied for early detection (5).
With recent technological advancements, saliva has been used to detect oral health and disease, cancer, salivary gland problems, and systemic disease. “Lab-on-a-chip” technology helps home diagnosis and increases healthcare success. The process of saliva collection is noninvasive; saliva is frequently collected for long-term disease monitoring without any pain or anxiety. Saliva sample handling is easier than blood sample handling. Moreover, salivary glands excretion contains proteins and is uniquely associated with saliva with sensitive and specific biomarkers than serum for certain oral diseases (6).
Specific salivary enzymes, cytokines, growth factors, metalloproteinases, endothelin, telomerase, cytokeratins, and mRNAs and DNA transcripts are utilized for diagnosis, prognosis, and post-therapy monitoring of disease (7).
This review article aimed to evaluate the available articles that employed saliva for the diagnosis of systemic diseases. For this purpose, a systematic search of the published literature (1990‒2016) was performed on Medline using PubMed. Several key words, including saliva, disease, systemic disease, health, and diagnosis, were used in this search. More than 100 articles were selected, and a review article was written about saliva and the diagnosis of systemic diseases (Figure 1).
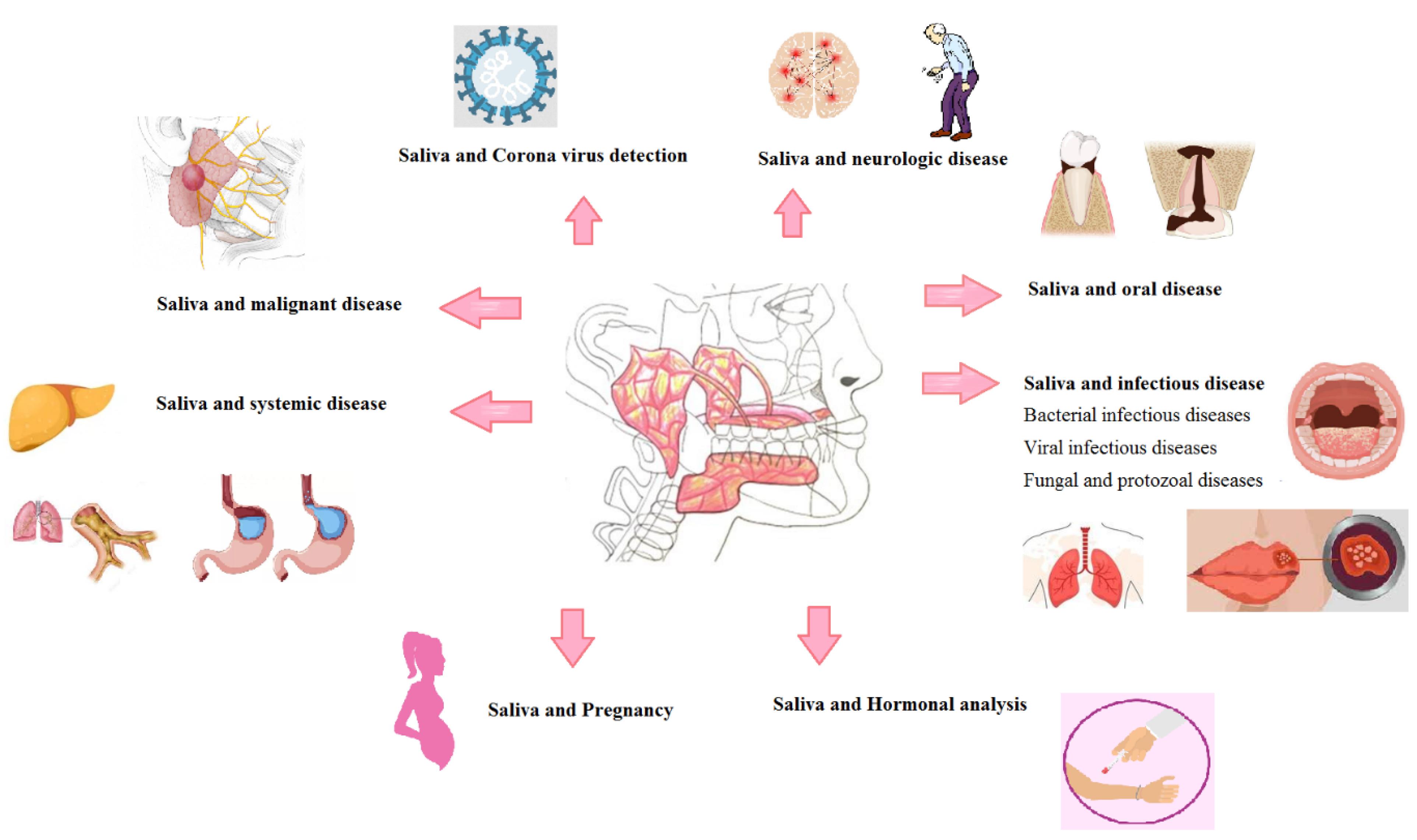
Figure 1.
Relationship between saliva and different systematic diseases
.
Relationship between saliva and different systematic diseases
Saliva and Oral Disease
Periodontal Disease
Saliva is increasingly applied for the diagnosis and monitoring of periodontal disease. Saliva enzyme activities (aspartate and alanine aminotransferases, lactate dehydrogenase, creatine kinase, alkaline, and acidic phosphates) were significantly increased in periodontitis patients. These biomarkers may be used in the diagnosis, prognosis, and monitoring of periodontal disease (8,9).Another study confirmed higher salivary amylase and secretory immunoglobulin A (IgA) in periodontitis patients (10). Alkaline phosphatase (ALP) plays an important role in bone loss. It was demonstrated that the levels of the ALP enzyme in the unstimulated saliva of periodontitis patients are higher than those of healthy individuals (11).
Elastase, α1-antitrypsin, α2-macroglobulin, and 3-hydroxy-fatty acids can be utilized as indicators of gingivitis and/or periodontitis. Albumin is another compound reported to be correlated with gingival inflammation. Periodontal diseases play a role in cardiovascular, cerebrovascular, and premature birth, so quick screening by saliva is highly important (12).
In spite of traditional periodontal diagnostic methods, nowadays, saliva enables prediction, detection, progression, and people’s risk for periodontitis (13).
Burning Mouth Syndrome
The salivary flow rate and total protein concentration of these patients were found to be lower, while the total mucin, potassium, chloride, and phosphate were higher than those of healthy ones (14).
Nerve growth factor and mass cell products are increased among these patients, while the amount of compounds such as substance P is decreased, and the number of neutrophil markers in these patients stays normal. These changes in the amount of the mentioned compounds can be employed for diagnosing the disease (15).
Oral Lichen Planus
The salivary amount of protein known as protein PLUNG (palate, lung, and nasal epithelium carcinoma-associated protein) is decreased in patients with lichen planus (16).
The concentrations of other salivary compounds, including interleukin 10 (IL-10), serum soluble tumor necrosis factor receptor 2, interferon alpha (INF-α), and INF-δ, are high in patients with erosive lichen planus (17‒19).
There has been a relationship between oxidative stress and lichen planus. Moreover, the level of antioxidants has been shown to be low in lichen planus patients. The amount of antioxidant vitamins, such as vitamins A, E, and C, was lower than normal in patients suffering from oral lichen planus (20).
Orthodontic Treatment
Secretory IgA (S-IgA) is regarded as an important indicator of the defensive status of the oral cavity. Implementing fixed or removable orthodontic appliances stimulates oral secretory immunity. In these cases, achieving an acceptable level of oral hygiene is extremely difficult, and the microflora and oral hemostasis undergo some changes (21).
Oral Leukoplakia
The salivary level of zinc (Zn) is significantly increased in patients with oral leukoplakia, which can be attributed to the increased metabolic requirement of Zn by dysplastic cells and tissue, which, in turn, demands a higher amount of Zn from the adjacent parts, including salivary secretion (22).
Recurrent Aphthous Stomatitis
RAS is the most common inflammatory ulceration of the oral cavity. Nowadays, oxidative stress is known as the main cause of RAS (23).
The imbalance of oxidant/antioxidant status may be the leading cause of tissue damage in patients suffering from RAS (23). One study demonstrated that the salivary epidermal growth factor was significantly reduced in patients suffering from RAS (15).
The results of another study showed that the salivary concentration of IgA in the active phases of RAS was significantly higher compared with that of the latent phases (24).
Endodontic
In the study by Ahmadi–Motamayel et al, the level of salivary alpha-amylase was significantly higher and associated with pain severity in patients with acute dental pulpitis. They concluded that alpha-amylase may be a good index for the objective assessment of pain intensity (25).
Dental Caries
Salivary flow rate, pH, calcium, protein, antioxidants, and buffering capacity play an important role in caries initiation and progression (26). Some salivary oligosaccharides, proteomic changes, proline-rich proteins, histatin 1, histatin S, and statin can predict dental caries (16). Total antioxidant capacity was higher in students with active caries, and antioxidant measurements may be helpful in caries activity detection and preventive dentistry (27‒29). A higher prevalence of caries was observed in patients with increased salivary streptococcus and lactobacilli numbers (30).
Saliva and Infectious Disease
Bacterial Infectious Diseases
Tuberculosis
Mycobacterium tuberculosis can be detected in the saliva by polymerase chain reaction (PCR) when it is in the acute phase with high levels of bacteria.Mycobacterium tuberculosis has been detected in the serum and saliva samples, which may pose dental healthcare workers with the risk of infection from blood -or saliva-borne pathogens (31). Alongside serology, anti-A60 IgA is another factor, and measuring its level in both the saliva and serum can help detect tuberculosis patients (32,33).
Pneumonia
C polysaccharides of Streptococcus pneumonia have been diagnosed in saliva without the detection of nucleic acids (10). IgE levels in saliva are increased prior to their increase in serum and may be used as a prognostic criterion in patients with bronchopulmonary pathology (34,35). One study demonstrated that a coated tongue is related to salivary bacterial count and the development of aspiration pneumonia (36). The risk of ventilator-associated pneumonia is higher among individuals who have higher dental plaque scores (37). A significant positive correlation was also observed between salivary bacteria and oral hygiene in dentate patients and pneumonia development (38,39).
Helicobacter pylori
Saliva is utilized as a diagnostic medium for the detection of H. pylori infection that causes diseases such as peptic ulcers or gastritis (1). Saliva is a reservoir for H. pylori (40). One-step saliva test which screens patients with dyspepsia without endoscopy, uses a monoclonal antibody that only reacts with H. pylori urease. Moreover, the oral antigen is significantly related to the serum antibody (41). H. pylori may accumulate in saliva and esophagus, and oral H. pylori might cause gastric reinfection after treatment (42).
Viral Infectious Diseases
Human Immunodeficiency Virus
Saliva is used for HIV 1 and 2 detections (10). Specific antibodies are utilized for the diagnosis of HIV in saliva as a first and quick test for HIV-1 detection with high sensitivity and specificity compared to the blood and urine tests. The level of salivary β2-microglobulin and TNF-α receptors can also be employed for HIV activity and acquired immunodeficiency syndrome-related inflammatory disease (1,6).
In cases where infected patients become symptomatic, the salivary IgA levels of HIV begin to decrease and are used as an indicator of the progression of HIV infection (26). Additionally, the level of lactoferrin in the saliva specimen of patients suffering from HIV infection would be reduced, whereas the rate of salivary peroxidase activity increases in such patients (43). In recent studies, salivary antioxidants have changed in HIV-positive patients (44-48).
Hepatitis
Saliva was sensitive and specific for viral hepatitis A, B, and C antigen detection and diagnosis, and commercial kits are now available for this purpose (1,6,34,49-51).
Rubella, Measles, and Mumps
The detection of IgM in saliva samples is used for the laboratory diagnosis of rubella. It has been illustrated that salivary samples contained specific IgM antibodies against rubella in more than 80% of cases, and the test specificity was as high as 96%. Samples taken from saliva have been employed for the detection of antibodies to the rubella and parotitis viruses (50,52).Saliva samples are useful in measles, mumps, and rubella infection detection and immunization (10).
Rotavirus
Salivary IgA is used as a biomarker for rotavirus infection and immune response (53).
Herpes Virus
Bell’s palsy can lead to the reactivation of infections caused by the herpes virus. It has been explained that this reactivation is detectable with the PCR of the virus in the saliva. HSV-1 shedding was observed in Bell’s palsy patients (10).
Dengue
Dengue is a viral disease with considerably different primary and secondary forms. The primary form can cause a febrile disease that is self-limiting. In contrast, the secondary form may result in hemorrhagic fever or dengue shock syndrome. The IgG level in saliva can be used to determine whether the disease is in its primary or secondary form (10,49).
One study demonstrated that the level of salivary IgA helps provide dengue early diagnosis. The high sensitivity of the technique makes it possible to be used in highly endemic areas in which most patients suffer from the secondary form of the infection (54).
Fungal and Protozoal Diseases
Candidiasis
Candidiasis is another infectious disease of the oral cavity. The disease is detectable by examining the presence of Candida spp. in the saliva.In case of the presence of the disease, there probably should be a high level of candidiasis saliva samples taken from an individual (55).
The colonization of Candidiasis in the oral mucosa leads to the proteomes of the salivary change. In this regard, the detection of proteins such as calprotectin, histatin 5, mucin, peroxidase, and high proline in saliva is of high importance in candidiasis diagnosis (15). One study reported that the level of salivary nitrates in patients suffering from such a disease significantly increased in patients with candidiasis (56).
Amebic Liver Abscess
The PCR technique showed salivary Entamoeba histolytica (97%) in amebic liver abscess cases; it would be a better sample comapred to blood for detecting E. histolytica (57).
Malaria
Saliva has Plasmodium DNA in malaria-infected individuals and could be used as an alternative specimen for diagnosis. Therefore, it is possible to detect malaria from samples taken from the urine and saliva using Cytb-PCR. This method has a more pleasant sensitivity than microscopic-based methods (58).
Saliva and Malignant Disease
Some biomarkers such as DNA, p53 gene and defensin-1, cancer antigen 15-3 (CA15-3), epidermal growth factor receptor, CD44 protein, carcinoembryonic antigen, a gastrointestinal cancer antigen, IL 8, thioredoxin, and c-erb B-2 have diagnostic properties in oral neoplasms, spinocellular carcinoma, breast cancer, and ovarian cancer. Early cancer diagnosis has a highly important role in prognosis (1,7,10,49,59-62). Salivary Cyfra 21-1, TPS, and CA125 increased in oral squamous cell carcinoma (OSCC) (7).
The amount of salivary antioxidants in OSCC patients was significantly higher in comparison with that of healthy people. In fact, an increased level of antioxidants in saliva is a remedial reaction to such a disease. Thus, administering such antioxidants can make it easier for saliva to fight against free radicals (63). Furthermore, it has been reported by several studies that the concentration of certain bacteria is increased in salivary specimens obtained from patients suffering from OSCC (64). These bacteria can play a role in OSCC and other similar diseases by up-regulating cytokines and other inflammatory mediators, thereby affecting complex metabolic pathways (65). Several studies have so far been conducted, demonstrating a significant correlation between candida and OSCC (66-68).
Wu et al first revealed the salivary proteome of the oral potentially malignant disorders (OMPD) group and pair-wisely analyzed the salivary proteomes between the OSCC and OPMD groups. Among the identified marker candidates, an increased level of RETN in saliva was highly correlated with the worse outcome of OSCC (60).
El-Naggar et al reported a loss of heterozygosity in saliva samples of 49% of oral cancer patients (69).
Saliva and Hematological Oncology
Bone marrow transplantation success can be estimated by the level of salivary neutrophils which arise earlier than blood. Oral mucositis improvement was also related to salivary neutrophil levels (3,70).
Saliva and Systemic Disease
Cardiovascular Diseases
Salivary amylase is used for cardiovascular surgery post-monitoring, and testing heart rate after stress (3,49).
Myoglobin, myeloperoxidase, and CRP have diagnostic abilities in myocardial infarction (15).
Kidney Dysfunction
The chemistry of saliva can change under not only chronic kidney disease but also its treatment. However, hemodialysis can reduce such changes to some extent (71).
The concentration and composition of some compounds present in saliva, such as proteins, sodium, and potassium, are the same as in plasma. It was shown that the concentrations of salivary calcium, phosphorous, urea, sodium, and potassium were higher in patients suffering from kidney dysfunction in comparison with healthy subjects. However, the concentration of bicarbonate did not differ significantly between these two groups (71,72). Because of its antimicrobial activities, urea can inhibit dental carries. Monitoring salivary urea and creatinineis useful in diagnosing renal damage and chronic renal failure in various stages of disease. The technique is simple, noninvasive, and rapid diagnostic (73).
Hypophosphatemia causes vascular calcification in patients with chronic renal failure. The swallowed salivary phosphate can also contribute to this problem. Since saliva is in perfect accordance with serum, phosphate can be a good marker for the initiation of treatment in this process. The standardization of saliva samples is of high importance when saliva is chosen as the research material because its composition may differ, to a considerable extent, from one individual to another or even within the same individual (74).
Nonerosive Reflux Disease
Many people all around the world suffer from gastroesophageal reflux disease (GERD) (75). Diagnostic methods normally used for detecting such a disease do not have high enough sensitivity and specificity, and some of them are invasive and expensive. Accordingly, there is an urgent need to develop new non-invasive, inexpensive methods with reliable sensitivity and specificity. Recent studies have shown that the concentration of salivary pepsin is higher among GERD patients, and its evaluation can help provide an office-based diagnosis (75).
Moreover, salivary bicarbonate secretion significantly increases in GERD patients, up to three times higher than its secretion in normal situations, to protect endoscopic mucosal damage; thus, it can be used as a biomarker for detecting or monitoring such a disease. However, more research is needed in this regard (75).
Diabetes Mellitus
Salivation can be stimulated by insulin, so the amount of saliva will decrease in patients suffering from diabetes mellitus (DM), and the probability of xerostomia will increase among such patients.
Protein concentration in the saliva of DM patients was related to the amount of hemoglobin A1C (HbA1c), so that the protein concentration is higher when HbA1c is higher. Furthermore, there is a significant association between the level of metabolic decompensation and the protein concentration in the saliva of these patients (76). Moreover, the salivary concentrations of amylase and IgA increase in such patients (74). pH can be used for detecting glandular dysfunction indicators in patients suffering from DM (76). The level of salivary antioxidants was changed in patients with DM (77).
Alcoholic Liver Cirrhosis
The parotid gland is enlarged in half of the patients, and the salivary flow rate is decreased to 50%. In addition, the salivary concentrations of other compounds such as sodium, bicarbonate, chlorine, and protein are decreased as well (72,74).
Crohn’s Disease
Salivary IL-1β, IL-6, and TNF-α increased in Crohn’s disease, and thus they can be considered as Crohn’s disease markers (78).
Cystic Fibrosis
There are several differences in the saliva of cystic fibrosis patients and healthy people. The saliva viscoelasticity would be increased in these patients, and the concentrations of some electrolytes and proteins differ in patient groups. Likewise, RNase activity is higher among cystic fibrosis patients. In these patients, insoluble calcium-protein complexes may be found as a result of the increase in the concentration of salivary calcium and total protein. Sodium, chloride, phosphate, lipid, epidermal growth factor, urea, and prostaglandin E2 are other compounds and molecules whose concentrations increase in the saliva of cystic fibrosis patients. Most of these compounds are highly effective in protecting teeth against caries (12,74). It is worth mentioning that the cathepsin D activity is higher among these patients than that among healthy individuals (79).
Multiple Sclerosis
The IgA production in these patients is significantly decreased during rest. It seems that until now, there has been no consensus on the use of salivary composition for diagnosing multiple sclerosis (72).
Saliva and Smoking
Apparently, smoking is regarded as an important source of oxidative stress. Losing the balance of free radical levels and reactive oxygen species with antioxidants may cause several inflammatory oral pathologies to begin and progress. Smoking can weaken the protective role of saliva by cutting down the salivary antioxidant concentration, such as superoxide dismutase (80,81).
Saliva and Pregnancy
Salivary flow rate, buffer, pH, calcium, and glucose were lower in pregnant women than in women with normal conditions. Salivary phosphate levels are increased. Such a condition in the oral cavity makes the situation suitable for the development of oral pathologies. During this period, the pregnant woman is highly vulnerable to dental caries because of the enhanced enamel demineralization and oral pathology (82).
Saliva and Hormonal Analysis
Free hormones presented in the saliva can be evaluated to obtain a view of the overall hormonal condition of the human body. Liposoluble hormones, which have low molecular weight, are easy to evaluate in the saliva. In contrast, hormones that are normally bonded to proteins, such as gonadotropins, prolactin, and thyrotropin, are more difficult to measure in samples taken from the saliva. Another important aspect of the free hormones in saliva is their potential use for detecting various cancers. It has been illustrated that the variation in the salivary levels of these hormones may be a sign of cancer progression and many hormonal diseases. Other hormones in the saliva consist of androstenedione, dihydrotestosterone, estradiol, and insulin (1,49,83,84).
Nowadays, the salivary estradiol level can predict preterm labor (84). An interesting feature of the test proposed and approved by the Food and Drug Administration for detecting estradiol is that the test is easy to administer so that it can be applied by women with a risk of premature or low-birth-weight babies (85).
Saliva and Neurologic Disease
Parkinson’s Disease
The progressive degeneration of monoaminergic and cholinergic neurons is the main characteristic of this disease. It is highly important to provide a reliable biomarker for diagnosing Parkinson’s disease (PD) in its earlier stages, monitor its progression, and assess its response to neuroprotective treatment (86).
The main pathological indicator of PD is the intra-neuronal inclusions of aggregated alpha-synuclein protein, which are also present in salivary glands and various sites of the autonomic system, playing a role in controlling saliva production in PD patients (87).
Tumilasci et al reported abnormal salivary composition in PD (88). It was demonstrated that the amount of the salivary flow rate and αsyn was decreased in PD patients, while the amount of Dj-1, cortisol, acetylcholinesterase activity, and total protein was increased (86).
Epilepsy
Gingival hypertrophy is possible in patients suffering from this disease, which requires a high level of oral hygiene. Moreover, these patients are exposed to the risk of selective IgA deficiency (72).
Alzheimer’s disease
Alzheimer’s disease (AD) early detection is highly important. Recently, it has been proposed that biopsy samples taken from salivary glands may be useful in predicting such a disease.
Nowadays, salivary biomarkers such as insulin-like growth factor-1, insulin-like growth factor-2, alpha-amylase, IL-1β, Aβ-40, Aβ-42, and TNF-α are used for AD diagnosis (89).
One study demonstrated that the p-rau/t-tau ratio is raised in AD patients, so the salivary level of this ratio can be employed as a marker for diagnosing AD patients (56). Lower salivary acetylcholinesterase and pseudocholinesterase were reported in AD (90).
Saliva and Autoimmune Disease
Sjogren’s syndrome affects exocrine glands and is diagnosed by several tests. Nonetheless, these tests have several drawbacks; they are invasive and expensive, and their accuracy in detecting the disease is not assured.
Some studies have proposed specific salivary cytokines such as lactoferrin, beta 2 microglobulin, lysozyme C, cystatin C, cystatin S, sodium, chloride, albumin, alpha 2 microglobulin, lipids, and inflammatory mediators such as eicosanoids, prostaglandin E2, thromboxane B2, and IL-2, IL-6, IgA, IgG, and IgM autoantibodies in disease diagnosis. Contrarily, the salivary concentration of some compounds would decrease in such patients, including amylase, carbonic anhydrase, and phosphate.
Kallikrein is another compound that has been found in salivary samples taken from patients suffering from Sjogren’s syndrome and rheumatoid arthritis. Accordingly, any variation in its salivary level can be used as a probable indicator for diagnosing the syndrome or monitoring its progression (1,49,72,91).
Graft-Versus-Host Disease
Graft-versus-host disease is a disorder that may occur after receiving a transplant surgery and results in the destruction of salivary glands, and thereby a significant reduction in salivary flow rate. The decrease in the salivary flow rate may be due to various causes, but when it lasts for more than 100 days after the surgery, it is a strong indicator of this disease. It should be noted that the concentrations of salivary sodium and lysozyme are increased in these patients, whereas the concentrations of phosphate and s-IgA are decreased in them (72).
Saliva as Diagnostic Testing of Medicines and Drugs
Samples taken from saliva are suitable for being utilized for detecting and monitoring non-ionizable drugs and those that are not affected by the salivary pH (4).
So far, saliva has been used to monitor the levels of lithium, carbamazepine, barbiturates, benzodiazepines, phenytoin, theophylline, cyclosporine, antipyrine, caffeine, cisplatin, diazepam, digoxin, ethosuximide, irinotecan, methadone, metoprolol, oxprenolol, paracetamol, pyrimidine, procainamide, quinine, sulfanilamide, and tolbutamide.
Therapeutic drug monitoring is normally applied to manage patients more appropriately. It can also be employed for testing people in prison. In this regard, samples taken from saliva can be highly useful because such samples demonstrate the free, non-protein bound, pharmacologically active component in the serum. Likewise, they can be used for drug abuse such as amphetamine, benzodiazepines, cocaine, ethanol, marijuana, nicotine, opioids, and phencyclidine (1,3,12). Further, saliva samples can also be applied to monitor a variety of drugs, including marijuana, cocaine, and alcohol (6).
Saliva and Genetic and Epigenetic Changes of Disease
The application of genomic DNA in forensic and clinical investigations is of high interest because of recent technological advancements in areas such as genome-wide microarrays (4). Nowadays, salivary DNA-based tests are extensively utilized in many laboratories and research centers for diagnosing mutations and polymorphisms associated with diseases (4).
Proteomic or genomic targets such as cytokines, enzymes, growth factors, metalloproteinase, endothelin, cytokeratins, telomerase, mRNAs, and DNA transcripts can be measured using saliva samples (92).The studies conducted on salivary mRNA profiling have revealed that there are four important biomarkers in patients suffering from oral cancer, including IL1-β, IL-8, ornithine decarboxylase antizyme 1, and spermidine N1 acetyltransferase (15). It is well documented that ncRNAs, a short version of RNAs, are normally stable almost in all body fluids (65); as a result, these compounds are being extensively studied for producing new target therapies and discovering new markers in body fluids such as saliva for diagnosing various diseases (93). Nowadays, there is a belief that ncRNAs may regulate various physiological functions. Moreover, the deregulation of ncRNAs, along with other molecular defects, contributes to the formation of cancer and tumor progression (68-70).
MiRNAs are a class of small non-coding RNA molecules, about 22 (18‒25 nt) nucleotides in length, that regulate protein expression and act as negative regulators of gene expression. The biological functions of miRNAs are well-defined; however, recent studies have explained that miRNAs and alterations in miRNA expression play an important role in the development and differentiation of various malignancies and have a tumor-suppressive function as well (94).
Moreover, these compounds contribute to almost all cellular processes that may modulate the malignant transformation of cells (95). Recent studies have demonstrated that these compounds can act as internal messengers and be a part of apoptotic bodies by being secreted and circulated in the blood. At least some of these extracellular miRNAs may act as a death signal in a competitive process against cancer cells (96).
Secretary miRNAs have several important advantages that make them useful as cancer biomarkers, including their high stability, high sensitivity in body fluids, convenience, and their potential for non-invasive diagnosis (94).
Plasma, saliva, breast milk, and seminal fluid have a higher number of detectable miRNA species. In contrast, urine, cerebrospinal fluid, and pleural fluid include far fewer detectable miRNA species (97).
Corona Virus Detection Through Saliva
The gold standard method for diagnosing Coronavirus is to perform reverse transcription quantitative PCR of samples obtained from oropharyngeal and nasopharyngeal swabs. The sensitivity of this method has been reported to be 66%–80%, and its specificity seems to be high, but false-positive results have been identified due to swab contamination, especially in asymptomatic patients. Additionally, many false-negative results have been identified in upper respiratory tract samples. While the swab nasopharyngeal technique is not an easy procedure, there is a risk of bleeding in patients with thrombocytopenia and infection of the sampler. Due to the need for a highly sensitive and specific diagnostic method for faster incubation in the majority of patients, saliva has been considered a rapid test and convenient alternative diagnostic method for COVID-19. RT-PCR is the most widely used method for the detection of COVID-19 by saliva, which showed a sensitivity of 84.9% and a specificity of 98.9%, which is higher than the swab method. In studies comparing saliva and blood samples, the levels of immunoglobulin observed in both were similar, suggesting that saliva could be a tool for short- and long-term humoral immunoassay in COVID-19 infection and evaluation of the efficacy of the corona vaccine (98-100).
Other Clinical Applications
Saliva has also been examined in several other systemic diseases such as salivary gland tumors, pancreatitis (74), anorexia, bulimia (101), autism, stem cell transplantation (102), pregnancy, smoking (103-105), and preterm and term newborns (106).
Depression is accompanied by reduced salivary proteins (107). One study described that variations in the psychoemotional state and psychological stresses may influence the biochemical composition of saliva, such as psychological stress, causing amylase elevation because of sympathetic activation (74).
Conclusion
Saliva is a complex and dynamic biological body fluid that is important for detecting physiological and pathological situations in the human body. In the last few years, saliva has gained increasing scientific interest not only for the extraction of various compounds (e.g., drugs, pollutants, and hormones in saliva) but also for the well-documented relation of saliva with bacterial, viral, and systemic diseases. Nonetheless, the concentrations of salivary biological markers are found at lower levels in comparison with plasma, and there are still not referenced values. As a diagnostic fluid, saliva has advantages over serum because of its non-invasive, simple, and easy collection method. Furthermore, saliva may provide a cost-effective approach for the screening of large populations.
Acknowledgements
We would like to express our sincere gratitude to all the individuals and organizations who contributed to the completion of this review article. Their support and guidance played a crucial role in shaping the content and ensuring its accuracy. Without their contributions, this work would not have been possible.
Authors’ Contribution
Conceptualization: Fatemeh Ahmadi Motamayel.
Data curation: Ali Mahdavinejad.
Formal analysis: Ali Mahdavinejad.
Funding acquisition: Setedeh Sareh Hendi, Fatemeh Ahmadi Motamayel.
Investigation: Seyedeh Sareh Hendi, Fatemeh Ahmadi Matamayel.
Methodology: Ali Mahdavi Nejad.
Project administration: Fatemeh Ahmadi Motamayel, Seyedeh Sareh Hendi.
Resources: Ali Mahdavi Nejad.
Software: Fatemeh Ahmadi Motamayel.
Supervision: Fatemeh Ahmadi Motamayel.
Validation: Ali Mahdavinejad.
Visualization: Seyedeh Sareh Hendi.
Writing–original draft: Fatemeh Ahmadi Motamayel, Seyedeh Sareh Hendi.
Writing–review & editing: Fatemeh Ahmadi Motamayel, Seyedeh Sareh Hendi.
Competing Interests
The authors declare no conflict of interests regarding the publication of this article. The information presented here is solely intended to provide an unbiased review of the current understanding of utilizing saliva biomarkers for diagnostic purposes.
Ethical Approval
This review article has been conducted in accordance with the ethical guidelines set forth by relevant institutions and regulatory bodies. The necessary approvals and permissions were obtained to ensure that the information presented here aligns with established ethical standards.
References
- Lima DP, Diniz DG, Moimaz SA, Sumida DH, Okamoto AC. Saliva: reflection of the body. Int J Infect Dis 2010; 14(3):e184-8. doi: 10.1016/j.ijid.2009.04.022 [Crossref] [ Google Scholar]
- Amerongen AV, Veerman EC. Saliva--the defender of the oral cavity. Oral Dis 2002; 8(1):12-22. doi: 10.1034/j.1601-0825.2002.1o816.x [Crossref] [ Google Scholar]
- Pink R, Simek J, Vondrakova J, Faber E, Michl P, Pazdera J. Saliva as a diagnostic medium. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2009; 153(2):103-10. doi: 10.5507/bp.2009.017 [Crossref] [ Google Scholar]
- Nunes LA, Mussavira S, Bindhu OS. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Biochem Med (Zagreb) 2015; 25(2):177-92. doi: 10.11613/bm.2015.018 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Davoodi P, Dalband M, Hendi SS. Saliva as a mirror of the body health. Avicenna J Dent Res 2010; 2(1):41-55. [ Google Scholar]
- Lee JM, Garon E, Wong DT. Salivary diagnostics. Orthod Craniofac Res 2009; 12(3):206-11. doi: 10.1111/j.1601-6343.2009.01454.x [Crossref] [ Google Scholar]
- Nagler RM. Saliva as a tool for oral cancer diagnosis and prognosis. Oral Oncol 2009; 45(12):1006-10. doi: 10.1016/j.oraloncology.2009.07.005 [Crossref] [ Google Scholar]
- Todorovic T, Dozic I, Vicente-Barrero M, Ljuskovic B, Pejovic J, Marjanovic M. Salivary enzymes and periodontal disease. Med Oral Patol Oral Cir Bucal 2006; 11(2):E115-9. [ Google Scholar]
- Moradi Haghgoo J, Soheilifar S, Bidgoli M, Rastegarfard N, Saremi M, Kafilzade S. Comparison of whole salivary lactate dehydrogenase level in patients with and without periodontal disease. Avicenna J Dent Res 2016; 8(4):e29026. doi: 10.17795/ajdr-29026 [Crossref] [ Google Scholar]
- Huang CM. Comparative proteomic analysis of human whole saliva. Arch Oral Biol 2004; 49(12):951-62. doi: 10.1016/j.archoralbio.2004.06.003 [Crossref] [ Google Scholar]
- Ketabi M, Mesripour M, Rafiei E. The comparison of GCF and salivary level of ALP in smokers, non-smokers with periodontitis and in healthy subjects. J Res Dent Sci 2015; 11(4):236-41. [ Google Scholar]
- Kaufman E, Lamster IB. The diagnostic applications of saliva--a review. Crit Rev Oral Biol Med 2002; 13(2):197-212. doi: 10.1177/154411130201300209 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Goodarzi MT, Jamshidi Z, Kebriaei R. Evaluation of salivary and serum antioxidant and oxidative stress statuses in patients with chronic periodontitis: a case-control study. Front Physiol 2017; 8:189. doi: 10.3389/fphys.2017.00189 [Crossref] [ Google Scholar]
- Hakeberg M, Hallberg LR, Berggren U. Burning mouth syndrome: experiences from the perspective of female patients. Eur J Oral Sci 2003; 111(4):305-11. doi: 10.1034/j.1600-0722.2003.00045.x [Crossref] [ Google Scholar]
- Taaheri J, Bakhshi M, Aryankia A, Noormohammadi R. Use of saliva for diagnosis of diseases. J Iran Dent Assoc 2014; 26(2):136-47. [ Google Scholar]
- Al Kawas S, Rahim ZH, Ferguson DB. Potential uses of human salivary protein and peptide analysis in the diagnosis of disease. Arch Oral Biol 2012; 57(1):1-9. doi: 10.1016/j.archoralbio.2011.06.013 [Crossref] [ Google Scholar]
- Ruochong W, Xuefeng Z, Siyu W. differential genotypes of TNF- and IL-10 for immonological diagnosis in discoid lupus erithematosus and oral lichen planus: A narrative review Front. Immunol 2022; 13:1-13. doi: 10.3389/fimmu.2022.967281 [Crossref] [ Google Scholar]
- Dan H, Liu W, Wang J, Wang Z, Wu R, Chen Q. Elevated IL-10 concentrations in serum and saliva from patients with oral lichen planus. Quintessence Int 2011; 42(2):157-63. [ Google Scholar]
- Ghallab NA, El-Wakeel N, Shaker OG. Levels of salivary IFN-gamma, TNF-alfa, and TNF receptor-2 as prognostic markers in (erosive) oral lichen planus. Mediators Inflamm 2010; 2010:847632. doi: 10.1155/2010/847632 [Crossref] [ Google Scholar]
- Abdolsamadi H, Rafieian N, Goodarzi MT, Feradmal J, Davoodi P, Jazayeri M. Levels of salivary antioxidant vitamins and lipid peroxidation in patients with oral lichen planus and healthy individuals. Chonnam Med J 2014; 50(2):58-62. doi: 10.4068/cmj.2014.50.2.58 [Crossref] [ Google Scholar]
- Youness SR, Hussein JS, Refaat W, El Hariri HM. Effect of orthodontic treatment on salivary immunoglobulin A levels among a group of healthy Egyptian children. IOSR J Dent Med Sci 2015; 14(4):58-63. doi: 10.9790/0853-14425863 [Crossref] [ Google Scholar]
- Kanneppady SK, Bhaskar A, Rao PK, Lakshman AR, Barua A, Kanneppady SS. Role of salivary zinc in oral leukoplakia–a biochemical study. Int J Innov Res Sci Eng Technol 2007; 4(2):44-7. doi: 10.15680/ijirset.2015.0402007 [Crossref] [ Google Scholar]
- Babaee N, Hosseinkazemi H, Pouramir M, Khakbaz Baboli O, Salehi M, Khadir F. Salivary oxidant/antioxidant status and hematological parameters in patients with recurrent aphthous stomatitis. Caspian J Intern Med 2016; 7(1):13-8. [ Google Scholar]
- Mohammad R, Halboub E, Mashlah A, Abou-Hamed H. Levels of salivary IgA in patients with minor recurrent aphthous stomatitis: a matched case-control study. Clin Oral Investig 2013; 17(3):975-80. doi: 10.1007/s00784-012-0785-2 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Shahriari S, Goodarzi MT, Moghimbeigi A, Jazaeri M, Babaei P. The relationship between the level of salivary alpha amylase activity and pain severity in patients with symptomatic irreversible pulpitis. Restor Dent Endod 2013; 38(3):141-5. doi: 10.5395/rde.2013.38.3.141 [Crossref] [ Google Scholar]
- Arunkumar S, Arunkumar JS, Krishna NB, Shakunthala GK. Developments in diagnostic applications of saliva in oral and systemic diseases-a comprehensive review. J Sci Innov Res 2014; 3(3):372-87. [ Google Scholar]
- Ahmadi-Motamayel F, Goodarzi MT, Hendi SS, Kasraei S, Moghimbeigi A. Total antioxidant capacity of saliva and dental caries. Med Oral Patol Oral Cir Bucal 2013; 18(4):e553-6. doi: 10.4317/medoral.18762 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Goodarzi MT, Mahdavinezhad A, Jamshidi Z, Darvishi M. Salivary and serum antioxidant and oxidative stress markers in dental caries. Caries Res 2018; 52(6):565-9. doi: 10.1159/000488213 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Hendi SS, Goodarzi MT. Evaluation of salivary lipid peroxidation end product level in dental caries. Infect Disord Drug Targets 2020; 20(1):65-8. doi: 10.2174/1871526519666181123182120 [Crossref] [ Google Scholar]
- Malathi N, Mythili S, Vasanthi HR. Salivary diagnostics: a brief review. ISRN Dent 2014; 2014:158786. doi: 10.1155/2014/158786 [Crossref] [ Google Scholar]
- Lee SA, Yoo SY, Kay KS, Kook JK. Detection of hepatitis B virus and Mycobacterium tuberculosis in Korean dental patients. J Microbiol 2004; 42(3):239-42. [ Google Scholar]
- Del Pezzo M, Alifano M, Faraone S, Battiloro R, De Pascalis R, Lavitola A. Detection of IgA against the mycobacterial antigen A60 in serum and saliva in patients with active pulmonary tuberculosis: preliminary results. New Microbiol 1996; 19(4):363-7. [ Google Scholar]
- Abdollahniya D, Hosseini SM, Kavyani Baghbaderani B, Mordadi A, Arabestani MR. Identification of Lactobacillus species isolated from traditional dairy products using RAPD-PCR. Avicenna J Clin Microbiol Infect 2018; 5(2):7-13. doi: 10.34172/ajcmi.2018.02 [Crossref] [ Google Scholar]
- Metel’skaia VA, Aleshkin BA, Voropaeva EA, Karaulov AV, Nesvizhskiĭ Iu V, Afanas’ev SS, et al. [Colonization resistance and immunological reactivity of children’s oropharyngeal mucosa in health and bronchopulmonary pathology]. Vestn Ross Akad Med Nauk. 2010(7):10-5. [Russian].
- Alikhani MY, Parsavash S, Arabestani MR, Hosseini SM. Prevalence of antibiotic resistance and class 1 integrons in clinical and environmental isolates of Pseudomonas aeruginosa. Avicenna J Clin Microbiol Infect 2017; 4(4):12086. doi: 10.5812/ajcmi.12086 [Crossref] [ Google Scholar]
- Abe S, Ishihara K, Adachi M, Okuda K. Tongue-coating as risk indicator for aspiration pneumonia in edentate elderly. Arch Gerontol Geriatr 2008; 47(2):267-75. doi: 10.1016/j.archger.2007.08.005 [Crossref] [ Google Scholar]
- Munro CL, Grap MJ, Elswick RK Jr, McKinney J, Sessler CN, Hummel RS 3rd. Oral health status and development of ventilator-associated pneumonia: a descriptive study. Am J Crit Care 2006; 15(5):453-60. [ Google Scholar]
- Abe S, Ishihara K, Adachi M, Okuda K. Oral hygiene evaluation for effective oral care in preventing pneumonia in dentate elderly. Arch Gerontol Geriatr 2006; 43(1):53-64. doi: 10.1016/j.archger.2005.09.002 [Crossref] [ Google Scholar]
- Sabouri Ghannad M, Hosseini SM, Kazemian H, Gharib A. Alzheimer’s disease and the role of infectious agents: a review. J Chem Pharm Sci 2016; 9(1):46-53. [ Google Scholar]
- Momtaz H, Souod N, Dabiri H, Sarshar M. Study of Helicobacter pylori genotype status in saliva, dental plaques, stool and gastric biopsy samples. World J Gastroenterol 2012; 18(17):2105-11. doi: 10.3748/wjg.v18.i17.2105 [Crossref] [ Google Scholar]
- Yang BL, Yeh C, Kwong WG, Lee SD. A novel one-step Helicobacter pylori saliva antigen test. J Chin Med Assoc 2015; 78(2):96-100. doi: 10.1016/j.jcma.2014.11.004 [Crossref] [ Google Scholar]
- Morales-Espinosa R, Fernandez-Presas A, Gonzalez-Valencia G, Flores-Hernandez S, Delgado-Sapien G, Mendez-Sanchez JL. Helicobacter pylori in the oral cavity is associated with gastroesophageal disease. Oral Microbiol Immunol 2009; 24(6):464-8. doi: 10.1111/j.1399-302X.2009.00541.x [Crossref] [ Google Scholar]
- Atkinson JC, Yeh C, Oppenheim FG, Bermudez D, Baum BJ, Fox PC. Elevation of salivary antimicrobial proteins following HIV-1 infection. J Acquir Immune Defic Syndr (1988) 1990; 3(1):41-8. [ Google Scholar]
- Ahmadi-Motamayel F, Vaziri-Amjad S, Goodarzi MT, Poorolajal J. Evaluation of salivary uric acid and pH in human immunodeficiency virus infected patients: a historical cohort study. Infect Disord Drug Targets 2018; 18(1):35-40. doi: 10.2174/1871526517666170428122405 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Vaziri-Amjad S, Davoodi P, Goodarzi MT, Poorolajal J. Evaluation of salivary alkaline phosphatase and albumin in HIV infected patients: a historical cohort study. Infect Disord Drug Targets 2019; 19(4):398-402. doi: 10.2174/1871526518666181005120804 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Vaziri-Amjad S, Goodarzi MT, Poorolajal J. Evaluation of salivary vitamin C and catalase in HIV positive and healthy HIV negative control group. Infect Disord Drug Targets 2017; 17(2):101-5. doi: 10.2174/1871526517666170116142547 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Vaziri-Amjad S, Goodarzi MT, Samie L, Poorolajal J. Evaluation of salivary melatonin levels in HIV-positive patients: a historical cohort study. Rev Recent Clin Trials 2017; 12(3):168-73. doi: 10.2174/1574887112666170725132528 [Crossref] [ Google Scholar]
- Vaziri-Amjad S, Davoodi P, Goodarzi MT, Abdolsamadi H, Poorolajal J, Parsa S. Salivary antioxidant and oxidative stress marker levels in HIV-positive individuals. Comb Chem High Throughput Screen 2019; 22(1):59-64. doi: 10.2174/1386207322666190306144629 [Crossref] [ Google Scholar]
- Llena-Puy C. The rôle of saliva in maintaining oral health and as an aid to diagnosis. Med Oral Patol Oral Cir Bucal 2006; 11(5):E449-55. [ Google Scholar]
- van der Eijk AA, Niesters HG, Götz HM, Janssen HL, Schalm SW, Osterhaus AD. Paired measurements of quantitative hepatitis B virus DNA in saliva and serum of chronic hepatitis B patients: implications for saliva as infectious agent. J Clin Virol 2004; 29(2):92-4. doi: 10.1016/s1386-6532(03)00092-1 [Crossref] [ Google Scholar]
- Zhang A, Sun H, Wang X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl Biochem Biotechnol 2012; 168(6):1718-27. doi: 10.1007/s12010-012-9891-5 [Crossref] [ Google Scholar]
- Bardow A, Moe D, Nyvad B, Nauntofte B. The buffer capacity and buffer systems of human whole saliva measured without loss of CO2. Arch Oral Biol 2000; 45(1):1-12. doi: 10.1016/s0003-9969(99)00119-3 [Crossref] [ Google Scholar]
- Jayashree S, Bhan MK, Kumar R, Raj P, Glass R, Bhandari N. Serum and salivary antibodies as indicators of rotavirus infection in neonates. J Infect Dis 1988; 158(5):1117-20. doi: 10.1093/infdis/158.5.1117 [Crossref] [ Google Scholar]
- Yap G, Sil BK, Ng LC. Use of saliva for early dengue diagnosis. PLoS Negl Trop Dis 2011; 5(5):e1046. doi: 10.1371/journal.pntd.0001046 [Crossref] [ Google Scholar]
- Coronado-Castellote L, Jiménez-Soriano Y. Clinical and microbiological diagnosis of oral candidiasis. J Clin Exp Dent 2013; 5(5):e279-86. doi: 10.4317/jced.51242 [Crossref] [ Google Scholar]
- Shi RT, Qin LZ, Xia DS, Deng DJ, Fan ZP, Shan ZC, et al. [Increase of saliva nitrate and nitrite level in patients with oral candidiasis]. Zhonghua Yu Fang Yi Xue Za Zhi 2009;43(7):607-10. [Chinese].
- Haque R, Kabir M, Noor Z, Rahman SM, Mondal D, Alam F. Diagnosis of amebic liver abscess and amebic colitis by detection of Entamoeba histolytica DNA in blood, urine, and saliva by a real-time PCR assay. J Clin Microbiol 2010; 48(8):2798-801. doi: 10.1128/jcm.00152-10 [Crossref] [ Google Scholar]
- Putaporntip C, Buppan P, Jongwutiwes S. Improved performance with saliva and urine as alternative DNA sources for malaria diagnosis by mitochondrial DNA-based PCR assays. Clin Microbiol Infect 2011; 17(10):1484-91. doi: 10.1111/j.1469-0691.2011.03507.x [Crossref] [ Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I, Rahimi A. Correlation of serum and salivary CA15-3 levels in patients with breast cancer. Med Oral Patol Oral Cir Bucal 2009; 14(10):e521-4. doi: 10.4317/medoral.14.e521 [Crossref] [ Google Scholar]
- Wu CC, Chu HW, Hsu CW, Chang KP, Liu HP. Saliva proteome profiling reveals potential salivary biomarkers for detection of oral cavity squamous cell carcinoma. Proteomics 2015; 15(19):3394-404. doi: 10.1002/pmic.201500157 [Crossref] [ Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I, Rahimi A, Seilanian-Toosi M. Correlation of serum and salivary CA125 levels in patients with breast cancer. J Contemp Dent Pract 2009; 10(6):E001-8. [ Google Scholar]
- Irani S. Diagnostic strategies for early detection of oral squamous cell carcinoma: a review article. Avicenna J Dent Res 2022; 14(3):137-43. doi: 10.34172/ajdr.2022.25 [Crossref] [ Google Scholar]
- Najafi S, Gholizadeh N, Manifar S, Rajabzadeh S, Kharazi Fard M. Salivary antioxidant level in oral squamous cell carcinoma. Iran J Blood Cancer 2015; 7(2):57-60. [ Google Scholar]
- Pushalkar S, Mane SP, Ji X, Li Y, Evans C, Crasta OR. Microbial diversity in saliva of oral squamous cell carcinoma. FEMS Immunol Med Microbiol 2011; 61(3):269-77. doi: 10.1111/j.1574-695X.2010.00773.x [Crossref] [ Google Scholar]
- Meurman JH. Oral microbiota and cancer. J Oral Microbiol 2010; 2(1):5195. doi: 10.3402/jom.v2i0.5195 [Crossref] [ Google Scholar]
- Uittamo J, Siikala E, Kaihovaara P, Salaspuro M, Rautemaa R. Chronic candidosis and oral cancer in APECED-patients: production of carcinogenic acetaldehyde from glucose and ethanol by Candida albicans. Int J Cancer 2009; 124(3):754-6. doi: 10.1002/ijc.23976 [Crossref] [ Google Scholar]
- Vadovics M, Ho J, Igaz N, Alföldi R, Rakk D, Veres É. Candida albicans enhances the progression of oral squamous cell carcinoma in vitro and in vivo. mBio 2021; 13(1):e0314421. doi: 10.1128/mBio.03144-21 [Crossref] [ Google Scholar]
- Sanketh DS, Patil S, Rao RS. Estimating the frequency of Candida in oral squamous cell carcinoma using calcofluor white fluorescent stain. J Investig Clin Dent 2016; 7(3):304-7. doi: 10.1111/jicd.12161 [Crossref] [ Google Scholar]
- El-Naggar AK, Mao L, Staerkel G, Coombes MM, Tucker SL, Luna MA. Genetic heterogeneity in saliva from patients with oral squamous carcinomas: implications in molecular diagnosis and screening. J Mol Diagn 2001; 3(4):164-70. doi: 10.1016/s1525-1578(10)60668-x [Crossref] [ Google Scholar]
- Akpek G, Knight RD, Wright DG. Use of oral mucosal neutrophil counts to detect the onset and resolution of profound neutropenia following high-dose myelosuppressive chemotherapy. Am J Hematol 2003; 72(1):13-9. doi: 10.1002/ajh.10250 [Crossref] [ Google Scholar]
- Anuradha BR, Katta S, Kode VS, Praveena C, Sathe N, Sandeep N. Oral and salivary changes in patients with chronic kidney disease: a clinical and biochemical study. J Indian Soc Periodontol 2015; 19(3):297-301. doi: 10.4103/0972-124x.154178 [Crossref] [ Google Scholar]
- Aps JK, Martens LC. Review: the physiology of saliva and transfer of drugs into saliva. Forensic Sci Int 2005; 150(2-3):119-31. doi: 10.1016/j.forsciint.2004.10.026 [Crossref] [ Google Scholar]
- Bader RS, Kora MA, El-Shalakany AH, Mashal BS. Clinical significance of saliva urea and creatinine levels in patients with chronic kidney disease. Menoufia Med J 2015; 28(2):406-10. [ Google Scholar]
- Castagnola M, Picciotti PM, Messana I, Fanali C, Fiorita A, Cabras T. Potential applications of human saliva as diagnostic fluid. Acta Otorhinolaryngol Ital 2011; 31(6):347-57. [ Google Scholar]
- Yandrapu H, Marcinkiewicz M, Poplawski C, Han K, Zbroch T, Goldin G. Role of saliva in esophageal defense: implications in patients with nonerosive reflux disease. Am J Med Sci 2015; 349(5):385-91. doi: 10.1097/maj.0000000000000443 [Crossref] [ Google Scholar]
- Aitken-Saavedra J, Rojas-Alcayaga G, Maturana-Ramírez A, Escobar-Álvarez A, Cortes-Coloma A, Reyes-Rojas M. Salivary gland dysfunction markers in type 2 diabetes mellitus patients. J Clin Exp Dent 2015; 7(4):e501-5. doi: 10.4317/jced.52329 [Crossref] [ Google Scholar]
- Fathi S, Borzouei S, Goodarzi MT, Poorolajal J, Ahmadi-Motamayel F. Evaluation of salivary antioxidants and oxidative stress markers in type 2 diabetes mellitus: a retrospective cohort study. Endocr Metab Immune Disord Drug Targets 2020; 20(4):584-90. doi: 10.2174/1871530319666191016103222 [Crossref] [ Google Scholar]
- Szczeklik K, Owczarek D, Pytko-Polończyk J, Kęsek B, Mach TH. Proinflammatory cytokines in the saliva of patients with active and non-active Crohn’s disease. Pol Arch Med Wewn 2012; 122(5):200-8. doi: 10.20452/pamw.1256 [Crossref] [ Google Scholar]
- Minarowska A, Minarowski L, Karwowska A, Sands D, Dabrowska E. The activity of cathepsin D in saliva of cystic fibrosis patients. Folia Histochem Cytobiol 2007; 45(3):165-8. [ Google Scholar]
- Abdolsamadi HR, Goodarzi MT, Mortazavi H, Robati M, Ahmadi-Motamayel F. Comparison of salivary antioxidants in healthy smoking and non-smoking men. Chang Gung Med J 2011; 34(6):607-11. [ Google Scholar]
- Golmohamadi MR, Abassi F, Esmaeili M, Jalayer Naderi N. Salivary pH and DMFT index in smokers and non-smokers: a comparative study based on the quantitative rate of smoking. Avicenna J Dent Res 2018; 10(4):140-2. doi: 10.34172/ajdr.2018.27 [Crossref] [ Google Scholar]
- Rio R, Azevedo Á, Simões-Silva L, Marinho J, Silva MJ, Sampaio-Maia B. The biochemistry of saliva throughout pregnancy. MedicalExpress (São Paulo) 2015; 2(5):M150506. doi: 10.5935/MedicalExpress.2015.05.06 [Crossref] [ Google Scholar]
- Santos-Pereira SA, Giraldo PC, Saba-Chujfi E, Amaral RL, Morais SS, Fachini AM. Chronic periodontitis and pre-term labour in Brazilian pregnant women: an association to be analysed. J Clin Periodontol 2007; 34(3):208-13. doi: 10.1111/j.1600-051X.2006.01038.x [Crossref] [ Google Scholar]
- Sindhu S, Jagannathan N. Saliva: a cutting edge in diagnostic procedures. J Oral Dis 2014; 2014:168584. doi: 10.1155/2014/168584 [Crossref] [ Google Scholar]
- McGregor JA, Jackson GM, Lachelin GC, Goodwin TM, Artal R, Hastings C. Salivary estriol as risk assessment for preterm labor: a prospective trial. Am J Obstet Gynecol 1995; 173(4):1337-42. doi: 10.1016/0002-9378(95)91383-1 [Crossref] [ Google Scholar]
- Fedorova T, Knudsen CS, Mouridsen K, Nexo E, Borghammer P. Salivary acetylcholinesterase activity is increased in Parkinson’s disease: a potential marker of parasympathetic dysfunction. Parkinsons Dis 2015; 2015:156479. doi: 10.1155/2015/156479 [Crossref] [ Google Scholar]
- Masters JM, Noyce AJ, Warner TT, Giovannoni G, Proctor GB. Elevated salivary protein in Parkinson’s disease and salivary DJ-1 as a potential marker of disease severity. Parkinsonism Relat Disord 2015; 21(10):1251-5. doi: 10.1016/j.parkreldis.2015.07.021 [Crossref] [ Google Scholar]
- Tumilasci OR, Cersósimo MG, Belforte JE, Micheli FE, Benarroch EE, Pazo JH. Quantitative study of salivary secretion in Parkinson’s disease. Mov Disord 2006; 21(5):660-7. doi: 10.1002/mds.20784 [Crossref] [ Google Scholar]
- Singhal RK, Anand S. Salivary Aβ-40, Aβ-42, IGF-I, IGF-II, alpha amylase, IL-1β, and TNF-alpha Alzheimer’s disease: a useful diagnostic tool. WebmedCentral NEUROSCIENCES 2014; 5(1):WMC004440. doi: 10.9754/journal.wmc.2014.004440 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Goodarzi MT, Tarazi S, Vahabian M. Evaluation of salivary acetylcholinesterase and pseudocholinesterase in patients with Alzheimer’s disease: a case-control study. Spec Care Dentist 2019; 39(1):39-44. doi: 10.1111/scd.12342 [Crossref] [ Google Scholar]
- Gotoh S, Watanabe Y, Fujibayashi T. Validity of stimulated whole saliva collection as a sialometric evaluation for diagnosing Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99(3):299-302. doi: 10.1016/j.tripleo.2004.09.016 [Crossref] [ Google Scholar]
- Li Y, St John MA, Zhou X, Kim Y, Sinha U, Jordan RC. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res 2004; 10(24):8442-50. doi: 10.1158/1078-0432.ccr-04-1167 [Crossref] [ Google Scholar]
- Majem B, Rigau M, Reventós J, Wong DT. Non-coding RNAs in saliva: emerging biomarkers for molecular diagnostics. Int J Mol Sci 2015; 16(4):8676-98. doi: 10.3390/ijms16048676 [Crossref] [ Google Scholar]
- Zhang J, Zhao H, Gao Y, Zhang W. Secretory miRNAs as novel cancer biomarkers. Biochim Biophys Acta 2012; 1826(1):32-43. doi: 10.1016/j.bbcan.2012.03.001 [Crossref] [ Google Scholar]
- Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ. The microRNA spectrum in 12 body fluids. Clin Chem 2010; 56(11):1733-41. doi: 10.1373/clinchem.2010.147405 [Crossref] [ Google Scholar]
- White NM, Fatoohi E, Metias M, Jung K, Stephan C, Yousef GM. Metastamirs: a stepping stone towards improved cancer management. Nat Rev Clin Oncol 2011; 8(2):75-84. doi: 10.1038/nrclinonc.2010.173 [Crossref] [ Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Hagiwara K, Takeshita F, Ochiya T. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J Biol Chem 2012; 287(2):1397-405. doi: 10.1074/jbc.M111.288662 [Crossref] [ Google Scholar]
- Ghosh S, Dhobley A, Avula KK, Joseph S, Gavali N, Sinha S. Role of saliva as a non-invasive diagnostic method for detection of COVID-19. Cureus 2022; 14(7):e27471. doi: 10.7759/cureus.27471 [Crossref] [ Google Scholar]
- Różański M, Walczak-Drzewiecka A, Witaszewska J, Wójcik E, Guziński A, Zimoń B. RT-qPCR-based tests for SARS-CoV-2 detection in pooled saliva samples for massive population screening to monitor epidemics. Sci Rep 2022; 12(1):8082. doi: 10.1038/s41598-022-12179-4 [Crossref] [ Google Scholar]
- Tee ML, Abrilla AA, Tee CA, Dalmacio LMM, Villaflor VJ 3rd, Abubakar AA. Saliva as alternative to naso-oropharyngeal swab for SARS-CoV-2 detection by RT-qPCR: a multicenter cross-sectional diagnostic validation study. Sci Rep 2022; 12(1):12612. doi: 10.1038/s41598-022-16849-1 [Crossref] [ Google Scholar]
- Spielmann N, Wong DT. Saliva: diagnostics and therapeutic perspectives. Oral Dis 2011; 17(4):345-54. doi: 10.1111/j.1601-0825.2010.01773.x [Crossref] [ Google Scholar]
- Imanguli MM, Atkinson JC, Harvey KE, Hoehn GT, Ryu OH, Wu T. Changes in salivary proteome following allogeneic hematopoietic stem cell transplantation. Exp Hematol 2007; 35(2):184-92. doi: 10.1016/j.exphem.2006.10.009 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Falsafi P, Abolsamadi H, Goodarzi MT, Poorolajal J. Evaluation of salivary antioxidants and oxidative stress markers in male smokers. Comb Chem High Throughput Screen 2019; 22(7):496-501. doi: 10.2174/1386207322666190806123616 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Falsafi P, Goodarzi MT, Poorolajal J. Comparison of salivary pH, buffering capacity and alkaline phosphatase in smokers and healthy non-smokers: retrospective cohort study. Sultan Qaboos Univ Med J 2016; 16(3):e317-21. doi: 10.18295/squmj.2016.16.03.009 [Crossref] [ Google Scholar]
- Ahmadi-Motamayel F, Falsafi P, Goodarzi MT, Poorolajal J. Evaluation of salivary catalase, vitamin C, and alpha-amylase in smokers and non-smokers: a retrospective cohort study. J Oral Pathol Med 2017; 46(5):377-80. doi: 10.1111/jop.12495 [Crossref] [ Google Scholar]
- Nemolato S, Messana I, Cabras T, Manconi B, Inzitari R, Fanali C. Thymosin beta(4) and beta(10) levels in pre-term newborn oral cavity and foetal salivary glands evidence a switch of secretion during foetal development. PLoS One 2009; 4(4):e5109. doi: 10.1371/journal.pone.0005109 [Crossref] [ Google Scholar]
- Grigoriev IV, Nikolaeva LV, Artamonov ID. Protein content of human saliva in various psycho-emotional states. Biochemistry (Mosc) 2003; 68(4):405-6. doi: 10.1023/a:1023695729019 [Crossref] [ Google Scholar]