Avicenna J Dent Res. 14(2):80-88.
doi: 10.34172/ajdr.2022.14
Review Article
Oral-Periodontal Health and Cytokine Storm: Correlation and Preventive Measures
Vanshika Jain 1  , Rizwana Mallick 2, *
, Rizwana Mallick 2, * 
Author information:
1BDS, Senior Research Fellow, Department of Dental Research and Implantology, Institute of Nuclear Medicine and Allied Sciences, Defence Research and Development Organization, New Delhi 110054, India
2BDS, MDS (Prosthodontics), PhD (Pursuing), Professor, Department of Prosthodontics and Crown and Bridge, Faculty of Dentistry Jamia Millia Islamia, New Delhi, India
Abstract
Coronavirus disease 19 (COVID-19) has taken the world by storm, affecting all age groups alike and presenting a plethora of signs and symptoms. Showcasing a high mortality rate, cytokine storm is identified as one of the most common culprits for death in affected individuals. In patients undergoing severe complications in the form of intubations and intensive care unit (ICU) admissions, increased cytokine levels have again been identified as a significant factor, indicating their substantial role in disease outcomes. Periodontitis, which is identified as a silent pandemic, is the most common oral disease that is found in individuals. The increased accumulations of plaques and calculus are the main causative agents, stimulating inflammatory cells in the periodontal tissue, leading to cytokine release. Individuals with the removable or fixed dental prosthesis are at increased risk of contracting fungal infections, which are also identified as increasing the cytokine levels and worsening an individual’s condition contracted with COVID-19. This review focuses on oral hygiene measures and scientifically proven aids that can be used by patients at home for reducing oral cytokine levels and the risk of COVID-19 related complications, thereby sensitizing them at a time when elective dental procedures are discouraged and patients are devoid of professional dental intervention. Mechanical removal of plaques and calculus cannot be substituted with auxiliary aids, but it is important that adjunct practices be adopted for efficient hygiene. Toothbrush hygiene should also be practiced to prevent disease progression and transmission. Adherence to these recommendations is not only required for healthy or infected individuals but also for viral infection recovered patients to avoid the possible risk of developing the black fungus infection.
Keywords: C-reactive protein, COVID-19, Cytokine release syndrome, Interleukins, Mycosis, Oral health
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Jain V, Mallick R. Oral-periodontal health and cytokine storm: correlation and preventive measures. Avicenna J Dent Res. 2022; 14(2):80-88. doi:10.34172/ajdr.2022.14
Introduction
Dating back to 2019 when the first coronavirus disease 2019 (COVID-19) patient was encountered in Wuhan, China, the disease has made its presence felt by taking the form of a pandemic (1). Looking at the potential means of human-to-human disease transmission, which is a secondary mode of transmission (animal-to-human is the primary one), droplet infection and surface contact (fomites) have been identified as the most common modes. However, the presence of the virus in urine and feces makes for potential feco-oral transmission as well; although none has been detected yet (2). Some other possible transmission routes include other bodily fluids and secretions such as saliva, tears, and semen, while vertical transmission has also been reported in rare instances (3).
Cytokines, which are used as a broad term for specific proteins released by immune and non-immune cells, are a group of auto-amplifying agents with either a pro- or an anti-inflammatory mechanism. From among the plethora of cytokines, interleukin-1 beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α) are the most notorious agents that are identified to play the most substantial role in inducing inflammatory responses (4,5).
Although there is no documented definition, a cytokine storm, also known as cytokine storm syndrome, is identified as an uncontrolled and unregulated release of excessive cytokines into the bloodstream in response to various triggers, including malignancy, auto-immune disorders, infections, and the like (6,7). Once in action, a cytokine storm causes the disruption of not only the pathological agent but also the physiologic body functions, ultimately, leading to acute respiratory distress syndrome and multiple-organ failure, if not managed timely and adequately (7,8).
After entering the human body through angiotensin-converting enzyme (ACE)-2 receptors, severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) disrupts their function by inducing cytokine storm, which is identified as the most prevalent etiology of the COVID-19 patient being transferred to intensive care units (ICUs), finally leading to their mortality (Figure 1). ACE2 receptors are most abundantly present in the lungs, thus causing them the maximum damage (9).
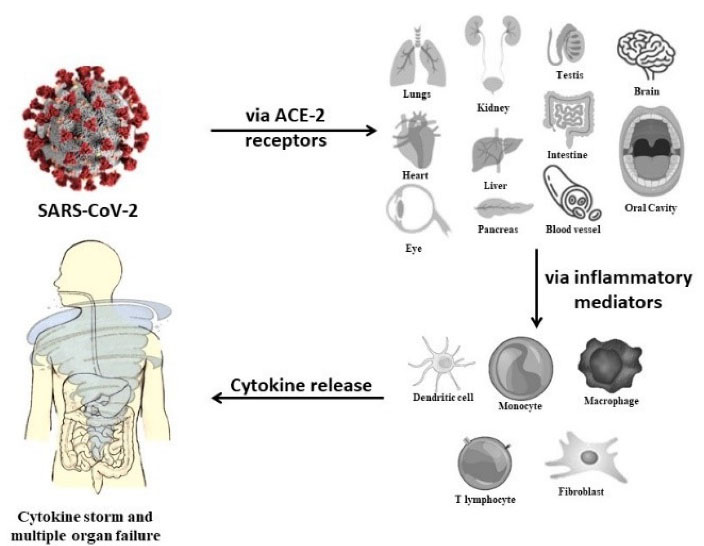
Figure 1.
SARS-CoV-2 Leading to Cytokine Storm. Note. ACE-2: Angiotensin-converting enzyme; SARS-CoV-2: Severe acute respiratory distress syndrome coronavirus 2.
.
SARS-CoV-2 Leading to Cytokine Storm. Note. ACE-2: Angiotensin-converting enzyme; SARS-CoV-2: Severe acute respiratory distress syndrome coronavirus 2.
While the focus for presence of ACE2 receptors in the lungs have been stressed upon, what is often ignored, and this may also act as a potential virus reservoir. Additionally, crevices and periodontal pockets provide a favorable niche for harboring and multiplication of the virus (10).
Thus, the presented paper aims at identifying the potential association between periodontal inflammatory markers and COVID-19 related complications and measures that can be adopted in this regard.
Methods
The presented scoping review was performed following the 20-point checklist of Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR) according to previous research (11). Electronic databases such as PubMed and Google Scholar were searched for articles published in the English language until May 2021 using a combination of keywords, including COVID-19, corona virus, cytokines, cytokine storm, COVID-19 complications, periodontal inflammatory markers, oral rinses, COVID and periodontal disease, and periodontal health. Controlled trials, case series, and case reports were included in this review study. However, papers published in languages other than English, review papers, and published literature not highlighting the role of cytokines in COVID-19 or the role of different oral hygiene agents in controlling periodontal inflammatory markers were excluded from this review.
COVID-19 and Periodontal Health
According to the 4th edition of the glossary of periodontal terms, periodontitis, which is identified as a silent pandemic, is defined as “inflammation of the supporting tissues of the teeth” (12). Plaques and calculus play a significant role in stimulating crevicular cytokine production (Figure 2). In addition to the increased amount of plaque and calculus deposits (in most cases) and increased bacterial load, periodontitis shows increased levels of inflammatory cytokines such as IL-1β, IL-6, IL-10, IL-12, interferon gamma (INF-γ), and the like. All these mediators, in turn, lead to soft and hard tissue destruction, which is often difficult to regenerate by regular treatment strategies (13). The increased levels of these cytokines eventually enter the bloodstream, inducing systemic functions by affecting the vascular system (14). Thus, as previously elucidated, there is enough evidence to indicate that COVID-19 infections follow a similar pathway as found during periodontal degeneration, thereby enhancing the chances of developing complications related to COVID-19 in individuals with the existing periodontal ailments and tested positive for COVID-19. In further support, the increased amounts of IL-6 have also been identified as a predictor of the development of respiratory illness and the need for ventilation (15). Further, the oral cavity is a potential reservoir of respiratory pathogens that aggravates the chances of a patient with poor periodontal status to develop community-acquired pneumonia (16).
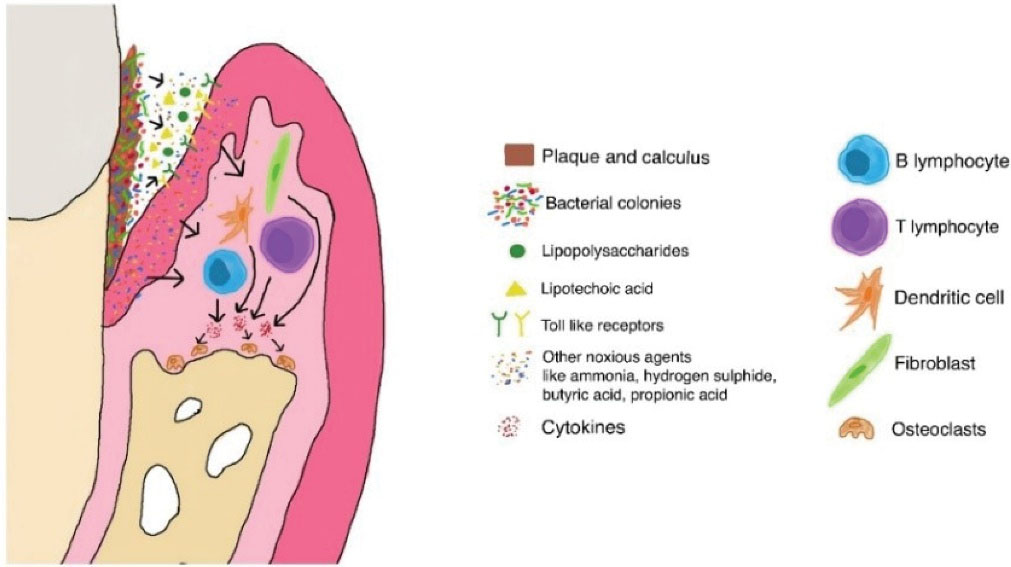
Figure 2.
Role of Plaques and Calculus in Stimulating Cytokines and Leading to Periodontal Destruction.
.
Role of Plaques and Calculus in Stimulating Cytokines and Leading to Periodontal Destruction.
Galectin-3 (Gal-3) is another pro-inflammatory mediator that is present in varied types of cells and has a structural similarity to spike the protein of COVID-19 which is essential for virus entry into the cell (17). The disruption of Gal-3 has been shown to decrease the levels of previously mentioned inflammatory ILs, thereby decreasing the complications.
In their case-control analysis, Marouf et al (18) found that patients, who tested positive for COVID-19 and had periodontitis, showed a higher incidence of developing COVID-19 related complications such as requiring ICU admission (odds ratio [OR] = 3.54, a 95% confidence interval [CI] = 1.39-9.05), needing assisted ventilation (OR = 4.57, 95% CI = 1.19-17.4), and death (OR = 8.81, 95% CI = 1.00-77.7). They also recorded an increased level of C-reactive protein (CRP), white blood cells, and D-dimer in these patients, further confirming their hypothesis of COVID-19 complications and periodontitis association. Likewise, Larvin et al reported a link between the increased risk of contracting COVID-19 infection in patients with periodontal complaints such as loose teeth, painful, and bleeding gums (19). In another study by Kamel et al (14) on COVID-19 recovered patients, a significantly strong inverse correlation was found between oral health and the severity of COVID-19 complications (P< 0.001, r = -0.512). They also reported similar results while comparing oral health with the required recovery period post COVID-19 infection and CRP levels (P < 0.001, r = -0.449 and P < 0.001, r = -0.190, respectively).
Periodontitis not only increases the mentioned inflammatory mediators but also acts as a risk factor for patients with hypertension, diabetes, and cardiovascular diseases, which are comorbidities that put individuals at a higher risk of COVID-19 complications (16,20,21). The raised CRP and other cytokine levels observed in the periodontitis patient not only put them at the risk of developing COVID-19 complications but can also form the basis of pathologic entity known as a persistent post-COVID-19 syndrome or long COVID-19 detected in recovered patients (22). With strong scientific evidence correlating pre-existing periodontitis to COVID-19 complications, it is important that stringent oral hygiene practices be followed so that to minimize the potential complications of the viral infection if contracted. Though presenting milder symptoms, cases of COVID-19 infection have been reported in individuals less than 18 years of age. Even in this age group, cases of critical morbidities have been reported, and thus it is important that good oral hygiene practice for children be ensured by the elders of the family (23,24).
In patients with dental implants, though it has been hypothesized that due to the absence of periodontal ligament cells around implants, IL-1 levels are lower in the peri-implant area compared to a healthy tooth gingival crevice, contrasting results have been put forth as well (25,26). Either way, it is evident that the concerned inflammatory markers are present in the peri-implant pocket and can be raised in the case of irritants. Therefore, it is important that extra efforts be made to keep the area clean and free of deposits. Moreover, maintaining oral hygiene with extra attention is important for patients using either partial or complete removable dentures or those with any form of fixed dental prosthesis other than implants as these factors increase the risk of deteriorated oral hygiene and deposits if not catered properly (27,28).
COVID-19 and Oral Fungal Infections
Candida albicans is a commensal of the oral cavity, the superficial infections of which are not restricted to immunocompromised hosts but are also commonly encountered in healthy individuals (29). Some in vitro studies involving C. albicans cultured on human oral epithelial cells collected from unstimulated saliva have shown the increased presence of pro-inflammatory markers and cytokines further adding to the gravity of COVID-19 complications (30-32). Oral fungal infections are encountered with an increased frequency in patients taking broad-spectrum antibiotics or those under corticosteroid therapy (29).
Although corticosteroid therapy is universally followed for all COVID-19 patients because of showing anti-inflammatory effects and reducing mortality, it further puts them at the risk of contracting fungal infections either during a hospital stay or post recovery (33). The presence of the mucormycosis (black fungus) infection involving the palate, paranasal sinuses, and orbital cavity, requiring extensive surgical treatment in COVID-19 recovered patients, has been increasingly reported by clinicians and surgeons in recent times (34-36). Recently concluded phase II trials have also indicated the use of inhaled budesonide in cases of mild COVID-19 infections (steroids in COVID-19) to decrease the likelihood of urgent medical care and hasten the patient’s recovery (37). The Indian Council of Medical Research has also recommended the drug’s use via a metered-dose inhaler or dry powder inhaler if the symptoms persist beyond 5 days (38). The increased chances of orofacial fungal infections in the form of candidiasis and mucormycosis with the use of corticosteroids have been well documented in the literature and thus call for reinforced oral hygiene measures (39,40).
Oral Hygiene Measures
Mechanical Plaque Control
Mechanical cleaning of the oral cavity remains the most effective mainstay for reducing oral tissue inflammation by decreasing plaque and calculus accumulation. The shutdown of elective dental procedures in the current pandemic situation deprives the patients of professional oral prophylaxis and thus stresses home care measures that can be adopted by individuals (41,42).
It is important that proper brushing techniques be followed to achieve effective cleaning. Among the various toothbrushing methods, the modified bass technique remains the most widely advocated method for adults by dental professionals, while the Fones method is popularized for the pediatric age groups (43). The use of powered toothbrushes should be encouraged in patients with a lack of manual dexterity and children who often avoid proper brushing of their teeth. These brushes show equal effectiveness in plaque removal as manual toothbrushes (44). Soft bristle toothbrushes and toothpaste should be employed twice a day for cleaning the teeth, investing two minutes at each time (45). Irrespective of the applied brushing method, it should be ensured that labial/buccal, lingual/palatal, and occlusal surfaces are touched while cleaning all surfaces of the tooth. The cleaning of interdental areas using dental floss or interdental brushes or end-tufted brushes and tongue cleaning should be stressed as well (46). Plaque disclosing agents can also be used by patients for better visualization and removal of plaques (47). Moreover, plaque-identifying toothpaste is now available aiding in enhanced plaque removal and CRP level reduction (48).
A home care oral irrigation device, also known as a water flosser or dental water jet, is a high-pressure irrigating device that helps in the efficient cleaning of interdental and subgingival areas that is otherwise difficult with regular mechanical methods. The use of the device along with regular oral hygiene measures has been shown to significantly improve periodontal health by reducing inflammatory markers (49). The use of a water flosser in addition to manual toothbrushing has been reported to be more effective in plaque control compared to string floss as an adjunct (50). Even a single-time use of the device has been found to reduce plaque scores by 89.09% in comparison to a reduction of 87.23% with the use of floss (51). The effectiveness of the irrigation device is further enhanced when employed with the 0.06% chlorhexidine (CHX) solution (52).
Toothbrush Care and Recommendations
Toothbrushes are a known niche for bacterial and viral colonies and can act as the potential source of re-infection in recovered patients (53). Thus, they should be properly handled and cleaned to avoid cross-contamination and infection spread.
-
The toothbrush should be regularly changed after 3 months. If the bristles start flaring before the intended duration, then, it should be changed earlier as frayed bristles trap more microorganisms (45,53).
-
In case of the development of any COVID-19 associated symptoms or awaiting test results of the real-time polymerase chain reaction test, one should discontinue the use of the current toothbrush and opt for a new one; a new toothbrush should again be opted for post recovery.
-
Patients with active COVID-19 infections should keep their toothbrushes separate from those of other inhabitants in case of a common usage place.
-
Toothbrushes should not be stored in the bathroom or closed containers; they should be dried before storing as wet bristles harbor more organisms (54).
-
They should be stored far enough from the sink to avoid the splashes of soap and water from hand washing and as far as possible from the toilet and sink. In case of limited space, they should be placed at least 4 feet from the toilet bowl and far enough from sink splashes (55).
-
In the case of multiple people using the same storage container, no two toothbrushes should touch each other.
-
Some liquids have been proven to be equally effective in the disinfection of toothbrushes, including 0.2% CHX, 0.1% sodium hypochlorite, 3% hydrogen peroxide, 0.05% cetylpyridinium chloride, Listerine, and 62%-71% ethanol (56-59). After use, the toothbrush should be thoroughly rinsed with water to remove any visible debris and remaining paste and placed in the disinfectant for 20 minutes, and then it should be stored in a container in an upright position for air drying (45). Chloroxylenol (DettolTM) is also effective with reduced efficiency (56).
Oral Rinses
The rinsing of the oral cavity using mouthwashes is one of the simplest adjunctive methods to control inflammatory mediator levels. A wide range of oral rinses have been studied in the literature serving the purpose of reducing inflammatory markers; however, each of them has certain limitations and thus needs to be selected based on an individual’s need. Popularly used mouthwashes include CHX (60) and povidone-iodine rinse (61). Other agents with potential use are 1.5% hydrogen peroxide (H2O2) rinse (62,63), turmeric rinse (64-66), simvastatin (SMV) mouthwash (67), and essential oil mouthwash (68,69). Further details about these mouthwashes are mentioned subsequently.
Chlorhexidine
A popularly used cationic substance, CHX, has been confirmed to be effective in the reduction of all previously mentioned inflammatory markers present in the gingival crevicular fluid, thereby acting as an effective adjunctive agent for plaque control (60). This can be due to the broad-spectrum effectiveness of the agent, along with high substantivity, providing a longer duration of anti-plaque activities (70). In a comparative analysis by Sharma et al, a highly significant decrease was noticed in IL-2 and INF-γ levels with the use of CHX mouthwash (P < 0.000). A significant decrease in their levels was also found with the use of povidone-iodine (P < 0.001) and essential oil mouthwashes (P < 0.013) (71). Similar results were reported in another study, indicating that curcumin-based mouthwash is equally effective in this regard (72). In addition to the anti-bacterial effects, CHX has anti-fungal and anti-viral properties (73).
Recommendations for CHX Rinse Use
CHX tends to chemically react with sodium lauryl sulphate and sodium monofluorophosphate, as two commonly encountered constituents of toothpaste. This reaction leads to a decrease in the efficacy of the oral rinse, and thus it is recommended to delay its use for at least 30 minutes post toothbrushing, whereas delaying its use by 2 hours completely neutralizes sodium lauryl sulphate and represents complete efficacy (74,75). Additionally, 15 mL rinse of 0.12% CHX containing 18 mg dose and 10 mL rinse of 0.2% CHX consisting of 20 mg dose are the two most commonly available concentrations; nonetheless, the former is the preferred concentration because of the dose-dependent staining by CHX and is also the food and drug administration approved composition (74,76). Due to the lack of human safety data on the CHX rinse in pregnant and lactating females, its use should be avoided if the benefits do not outweigh the risk (77). Varying amounts of altered taste perceptions have been observed with the use of the CHX rinse, thus it is advised not to continue its use for a long duration; however, no published literature has yielded numerical values of this duration (70,78).
Povidone Iodine (PVP-I) Rinse
PVP-I, also known as betadine, is a halogen-based compound with an activity against a broad range of pathogens, including fungi and viruses. This vast range of the action, including the one against the oral biofilm, makes it a useful agent for decreasing oral inflammatory markers (61). Different concentrations of the formulation are available to be used as an oral rinse (63). According to the results of in-vivo studies, 0.23% concentration could reduce the levels of the SARS-CoV-2 virus and have an in-vitro efficacy equivalent to that of 70% ethanol (79). A 10% solution of PVP-I, which is equivalent to 1% available iodine, has been included in the list of essential medicines by the World Health Organization (80). Long-term studies have revealed that 0.1% concentration is equally effective in improving the periodontal status (61,81). There has been no report on the detrimental effect of the rinse on thyroid function; however, there might be incidences of teeth and mucosa staining, taste alteration, and rarely allergic reactions (61,82). The use of the rinse can be safely employed for both adults and children. A 10% concentration is mostly preferred for use, and the gargle should be expectorated after rinsing rather than being swallowed (61). Approximately 9 mL of the rinse should be utilized at one time for rinsing (83).
Other Oral Rinses
The use of 1.5% H2O2 rinse stabilized with erythritol and glycerin for reducing gingival inflammation has been reported to be equally effective or slightly lesser efficient than CHX and have anti-bacterial, anti-viral, and anti-plaque effects (62,63), helping in reducing the inflammatory markers. The chemical agent has oxidizing properties that help in controlling dental plaques without causing damage to adjacent tissues (84). A combined formulation containing 0.12% CHX and 1.5% H2O2 has been demonstrated to be more palatable compared to the individual use of the former without causing any fall in anti-bacterial properties, while the sequential use of the two agents has also represented satisfactory results in reducing plaque deposits (85,86). Furthermore, a 3% concentration of H2O2 can be effectively used, but percentages beyond the mentioned one should not be utilized in this regard (83). It is advisable to take 10-15 mL of solution at a time for the thorough rinsing of the oral cavity at a time (83).
Turmeric, which is derived from Curcuma longa, has been employed in Indian and Chinese traditional medicine and is found to have anti-inflammatory, anti-bacterial, and anti-oxidant properties (87). Based on reports, the use of 0.1% turmeric mouthwash is equally efficacious to the 0.2% CHX rinse in controlling plaque accumulation and reducing plaque and gingival index (PI and GI) scores (64-66). In-vitro studies have confirmed the activity of the agents against fungal species as well; however, there is no evidence of the human in-vivo application in this respect (88,89).
Statins, commonly applied cholesterol-reducing systemic agents, have shown viable results in reducing the virus entry into the host cell. When used for the management of periodontal diseases, they represent positive results in reducing periodontal pathogens. It should be noted that 1% SMV mouthwash is the most frequent concentration employed for oral rinses (67). The solution can be made by dissolving a 20 mg tablet of SMV in distilled water; however, additive flavoring agents might be required due to a non-palliative taste. Varying concentrations of SMV in the range of 0.6%-1.8% and its combination with CHX have revealed favorable results in periodontal assessment parameters such as bleeding on probing and probing depth, along with a reduction in inflammatory cytokines (90). In addition, 1.2 mg SMV has been reported to reduce raised periodontal IL-6 levels (91); further, SMV is available in gel form and has equally efficacious results (92).
Listerine® mouthwash, which contains essential oils, is an easily available over-the-counter agent that is found to be effective in reducing plaque deposits and CRP levels (68,69). Alcohol-containing mouthwashes have anti-inflammatory, anti-bacterial, anti-fungal, and anti-viral characteristics; however, the range of their disadvantages discourages their use (70,93).
Topical Gels
Based on the findings, 1% weight/weight (w/w) CHX gels and 10 mg metronidazole gels or a combination of the two gels could significantly reduce plaque accumulation subsequently reporting reduced values for assessed GI after 6, 12, and 24 weeks of regular use (94). However, there are reported incidences of altered taste and tooth discoloration after the use of CHX-based gels. The results of another study comparing the efficacy of 4 different gels (curcumin-, CHX-, PVP-I-, and metronidazole based gels) in reducing gingivitis showed an insignificant difference with respect to plaque index scores; however, a substantial reduction in GI scores was reported with CHX gels after 3 weeks of use (95).
Limitations
The presented work is a scoping review based on the preliminary studies conducted in the field of the impact of oral health on potential complications of COVID-19 infections and the measures patients can take to maintain and improve their oral health when elective dental care is inaccessible. A scoping review only points towards the measures that should be taken and help guide towards a path for further research. Thus, it is encouraged to undertake more clinical studies and conduct a systematic review of the same type so that to obtain conclusive scientific evidence in this regard.
Suggestions and Conclusion
-
While no measure other than following precautions in the form of using masks, sanitizer, and maintaining social distancing can reduce the risk of contracting the COVID-19 infection, it is important that strict oral hygiene be maintained to reduce the risk of complications associated with the contraction of the viral disease.
-
Increased oral cytokine levels put the patient at the risk of COVID-19 complications if not only restricted to patients with compromised health but extend to patients with any kind or removable or fixed prosthesis, including dental implants.
-
Use of interdental cleaning aids, mouthwashes, or topical gels should be adopted as an adjunct to toothbrushing rather than considering it as a substitute for plaque control.
-
CHX is the gold standard agent and should be preferred unless contraindicated.
-
Although 9 mL of 10% PVP-I is an effective oral rinse, there may be rare instances of allergic reactions and thus should be given due notice by the user.
-
Overall, 15 mL of 1.5% H2O2 is an effective substitute for CHX for patients who are intolerant to the latter.
-
Use of alcohol-based rinses should be avoided because of their potential side effects.
-
While using any type of mouthwash, at least 1 minute, equally divided between the rinsing of the oral cavity and the back of the throat, should be devoted at a time.
-
A water flosser is an effective interdental cleaning aid the use of which should be encouraged and brought into day-to-day oral hygiene practices.
-
Stringent oral hygiene should not only be followed to avoid COVID-19 complications but also should be adhered to even post COVID-19 recovery.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Ethical Statement
Not applicable.
References
- Bhandari A, Jain V, Bhandari R. COVIDentistry: combating corona virus spread in dental setup: Indian prospective. Def Life Sci J 2021; 6(1):93-105. doi: 10.14429/dlsj.6.16190 [Crossref] [ Google Scholar]
- Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions#:~:text=TransmissionofSARS-CoV,%2Ctalksorsings. Accessed April 23, 2021.
- Karia R, Gupta I, Khandait H, Yadav A, Yadav A. COVID-19 and its modes of transmission. SN Compr Clin Med 2020; 2(10):1798-801. doi: 10.1007/s42399-020-00498-4 [Crossref] [ Google Scholar]
- Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci 2019; 20(23):6008. doi: 10.3390/ijms20236008 [Crossref] [ Google Scholar]
- Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007; 45(2):27-37. doi: 10.1097/AIA.0b013e318034194e [Crossref] [ Google Scholar]
- Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol 2020; 11:1708. doi: 10.3389/fimmu.2020.01708 [Crossref] [ Google Scholar]
- Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020; 383(23):2255-73. doi: 10.1056/NEJMra2026131 [Crossref] [ Google Scholar]
- Iannaccone G, Scacciavillani R, Del Buono MG, Camilli M, Ronco C, Lavie CJ. Weathering the cytokine storm in COVID-19: therapeutic implications. Cardiorenal Med 2020; 10(5):277-87. doi: 10.1159/000509483 [Crossref] [ Google Scholar]
- Mustafa MI, Abdelmoneim AH, Mahmoud EM, Makhawi AM. Cytokine storm in COVID-19 patients, its impact on organs and potential treatment by QTY code-designed detergent-free chemokine receptors. Mediators Inflamm 2020; 2020:8198963. doi: 10.1155/2020/8198963 [Crossref] [ Google Scholar]
- Troeltzsch M, Berndt R, Troeltzsch M. Is the oral cavity a reservoir for prolonged SARS-CoV-2 shedding?. Med Hypotheses 2021; 146:110419. doi: 10.1016/j.mehy.2020.110419 [Crossref] [ Google Scholar]
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169(7):467-73. doi: 10.7326/m18-0850 [Crossref] [ Google Scholar]
- Glossary of Periodontal Terms. 4th ed. 2001. Chicago.
- Ramadan DE, Hariyani N, Indrawati R, Ridwan RD, Diyatri I. Cytokines and chemokines in periodontitis. Eur J Dent 2020; 14(3):483-95. doi: 10.1055/s-0040-1712718 [Crossref] [ Google Scholar]
- Kamel AHM, Basuoni A, Salem ZA, AbuBakr N. The impact of oral health status on COVID-19 severity, recovery period and C-reactive protein values. Br Dent J. 2021:1-7. 10.1038/s41415-021-2656-1.
- Molayem S, Pontes CC. The mouth-COVID connection: IL-6 levels in periodontal disease—potential role in COVID-19-related respiratory complications. J Calif Dent Assoc. 2020. 10.35481/jcda-48-10-01.
- Botros N, Iyer P, Ojcius DM. Is there an association between oral health and severity of COVID-19 complications?. Biomed J 2020; 43(4):325-7. doi: 10.1016/j.bj.2020.05.016 [Crossref] [ Google Scholar]
- Kara C, Çelen K, Dede F, Gökmenoğlu C, Kara NB. Is periodontal disease a risk factor for developing severe COVID-19 infection? The potential role of Galectin-3. Exp Biol Med (Maywood) 2020; 245(16):1425-7. doi: 10.1177/1535370220953771 [Crossref] [ Google Scholar]
- Marouf N, Cai W, Said KN, Daas H, Diab H, Chinta VR. Association between periodontitis and severity of COVID-19 infection: a case-control study. J Clin Periodontol 2021; 48(4):483-91. doi: 10.1111/jcpe.13435 [Crossref] [ Google Scholar]
- Larvin H, Wilmott S, Wu J, Kang J. The impact of periodontal disease on hospital admission and mortality during COVID-19 pandemic. Front Med (Lausanne) 2020; 7:604980. doi: 10.3389/fmed.2020.604980 [Crossref] [ Google Scholar]
- People with Certain Medical Conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed April 25, 2021.
- Lee JH, Choi JK, Jeong SN, Choi SH. Charlson comorbidity index as a predictor of periodontal disease in elderly participants. J Periodontal Implant Sci 2018; 48(2):92-102. doi: 10.5051/jpis.2018.48.2.92 [Crossref] [ Google Scholar]
- Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, Lybeck M, et al. A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol. 2021:1-9. 10.1007/s12016-021-08848-3.
- Coronavirus Disease (COVID-19): Schools. World Health Organization. https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-schools. Accessed April 25, 2021.
- Children and COVID-19: State-Level Data Report. American Academy of Pediatrics. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/. Accessed April 25, 2021.
- Ketabi AR, Friese H, Weber H. A comparison of IL-1β, E2 (PGE2), PAI-2 and TNF-α levels in gingival crevicular fluid (GCf) and peri-implant crevicular fluid (PICF) from groups of patients with healthy teeth, healthy implants, periodontitisand peri-implantitis. Dentistry 2017; 7(8):445. doi: 10.4172/2161-1122.1000445 [Crossref] [ Google Scholar]
- Shama MM, Aboukhadr M, Madi M, Abdelhady S. Comparison Between level of intereukin 10 in the gingival crevicular fluid and peri-implant sulcular fluid around healthy dental implants (split mouth study). Alex Dent J 2016; 41(1):26-30. doi: 10.21608/adjalexu.2016.59168 [Crossref] [ Google Scholar]
- Turgut Cankaya Z, Yurdakos A, Gokalp Kalabay P. The association between denture care and oral hygiene habits, oral hygiene knowledge and periodontal status of geriatric patients wearing removable partial dentures. Eur Oral Res 2020; 54(1):9-15. doi: 10.26650/eor.20200048 [Crossref] [ Google Scholar]
- Szalewski L, Pietryka-Michałowska E, Szymańska J. Oral hygiene in patients using removable dentures. Polish J Public Health 2017; 127(1):28-31. [ Google Scholar]
- Rajendra Santosh AB, Muddana K, Bakki SR. Fungal infections of oral cavity: diagnosis, management, and association with COVID-19. SN Compr Clin Med. 2021:1-12. 10.1007/s42399-021-00873-9.
- Steele C, Fidel PL Jr. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun 2002; 70(2):577-83. doi: 10.1128/iai.70.2.577-583.2002 [Crossref] [ Google Scholar]
- Ashman RB, Vijayan D, Wells CA. IL-12 and related cytokines: function and regulatory implications in Candida albicans infection. Clin Dev Immunol 2011; 2011:686597. doi: 10.1155/2011/686597 [Crossref] [ Google Scholar]
- Diesch T, Filippi C, Fritschi N, Filippi A, Ritz N. Cytokines in saliva as biomarkers of oral and systemic oncological or infectious diseases: a systematic review. Cytokine 2021; 143:155506. doi: 10.1016/j.cyto.2021.155506 [Crossref] [ Google Scholar]
- Coronavirus Disease (COVID-19): Dexamethasone. World Health Organization. https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-Recommendation1%3AWHOstrongly,medicationforanothercondition. Accessed May 2, 2021.
- Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus 2020; 12(9):e10726. doi: 10.7759/cureus.10726 [Crossref] [ Google Scholar]
- Roy M. Dual infection in a COVID-19 patient – mucormycosis and actinomycosis. Saudi J Pathol Microbiol 2021; 6(1):53-5. doi: 10.36348/sjpm.2021.v06i01.010 [Crossref] [ Google Scholar]
- Pauli MA, Pereira LM, Monteiro ML, de Camargo AR, Rabelo GD. Painful palatal lesion in a patient with COVID-19. Oral Surg Oral Med Oral Pathol Oral Radiol 2021; 131(6):620-5. doi: 10.1016/j.oooo.2021.03.010 [Crossref] [ Google Scholar]
- Ramakrishnan S, Nicolau DV, Jr Jr. , Langford B, Mahdi M, Jeffers H, Mwasuku C, et al Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med 2021; 9(7):763-72. doi: 10.1016/s2213-2600(21)00160-0 [Crossref] [ Google Scholar]
- Home Isolation & Care for COVID-19. Indian Council of Medical Research. https://www.icmr.gov.in/pdf/covid/techdoc/COVID_HOME_CARE.pdf. Accessed May 7, 2021.
- Godara N, Godara R, Khullar M. Impact of inhalation therapy on oral health. Lung India 2011; 28(4):272-5. doi: 10.4103/0970-2113.85689 [Crossref] [ Google Scholar]
- Ayinampudi BK, Gannepalli A, Pacha VB, Kumar JV, Khaled S, Naveed MA. Association between oral manifestations and inhaler use in asthmatic and chronic obstructive pulmonary disease patients. J Dr NTR Univ Health Sci 2016; 5(1):17-23. doi: 10.4103/2277-8632.178950 [Crossref] [ Google Scholar]
- Liu C, Zhang S, Zhang C, Tai B, Jiang H, Du M. The impact of coronavirus lockdown on oral healthcare and its associated issues of pre-schoolers in China: an online cross-sectional survey. BMC Oral Health 2021; 21(1):54. doi: 10.1186/s12903-021-01410-9 [Crossref] [ Google Scholar]
- Walter E, von Bronk L, Hickel R, Huth KC. Impact of COVID-19 on dental care during a national lockdown: a retrospective observational study. Int J Environ Res Public Health 2021; 18(15):7963. doi: 10.3390/ijerph18157963 [Crossref] [ Google Scholar]
- Wainwright J, Sheiham A. An analysis of methods of toothbrushing recommended by dental associations, toothpaste and toothbrush companies and in dental texts. Br Dent J 2014; 217(3):E5. doi: 10.1038/sj.bdj.2014.651 [Crossref] [ Google Scholar]
- Deery C, Heanue M, Deacon S, Robinson PG, Walmsley AD, Worthington H. The effectiveness of manual versus powered toothbrushes for dental health: a systematic review. J Dent 2004; 32(3):197-211. doi: 10.1016/j.jdent.2003.11.006 [Crossref] [ Google Scholar]
- Oral Health Topics: Toothbrushes. American Dental Association. https://www.ada.org/en/member-center/oral-health-topics/toothbrushes. Accessed May 7, 2021.
- Mandal A, Singh DK, Siddiqui H, Das D, Dey AK. New dimensions in mechanical plaque control: an overview. Indian J Dent Sci 2017; 9(2):133-9. doi: 10.4103/ijds.ijds_18_17 [Crossref] [ Google Scholar]
- Nepale MB, Varma S, Suragimath G, Abbayya K, Zope S, Kale V. A prospective case-control study to assess and compare the role of disclosing agent in improving the patient compliance in plaque control. J Oral Res Rev 2014; 6(2):45-8. doi: 10.4103/2249-4987.152907 [Crossref] [ Google Scholar]
- Fasula K, Evans CA, Boyd L, Giblin L, Belavsky BZ, Hetzel S. Randomized Trial of Plaque-Identifying Toothpaste: Decreasing Plaque and Inflammation. Am J Med 2017; 130(6):746-9. doi: 10.1016/j.amjmed.2016.09.003 [Crossref] [ Google Scholar]
- Cutler CW, Stanford TW, Abraham C, Cederberg RA, Boardman TJ, Ross C. Clinical benefits of oral irrigation for periodontitis are related to reduction of pro-inflammatory cytokine levels and plaque. J Clin Periodontol 2000; 27(2):134-43. doi: 10.1034/j.1600-051x.2000.027002134.x [Crossref] [ Google Scholar]
- Goyal CR, Lyle DM, Qaqish JG, Schuller R. Evaluation of the plaque removal efficacy of a water flosser compared to string floss in adults after a single use. J Clin Dent 2013; 24(2):37-42. [ Google Scholar]
- Abdellatif H, Alnaeimi N, Alruwais H, Aldajan R, Hebbal MI. Comparison between water flosser and regular floss in the efficacy of plaque removal in patients after single use. Saudi Dent J 2021; 33(5):256-9. doi: 10.1016/j.sdentj.2021.03.005 [Crossref] [ Google Scholar]
- Bunk D, Eisenburger M, Häckl S, Eberhard J, Stiesch M, Grischke J. The effect of adjuvant oral irrigation on self-administered oral care in the management of peri-implant mucositis: a randomized controlled clinical trial. Clin Oral Implants Res 2020; 31(10):946-58. doi: 10.1111/clr.13638 [Crossref] [ Google Scholar]
- Frazelle MR, Munro CL. Toothbrush contamination: a review of the literature. Nurs Res Pract 2012; 2012:420630. doi: 10.1155/2012/420630 [Crossref] [ Google Scholar]
- Yadav S. Toothbrushes in bathroom-clean before you clean. J Adv Med Dent Sci Res 2015; 3(5):S57-S59. [ Google Scholar]
- Safe Storage for Family Toothbrushes. https://www.colgate.com/en-in/oral-health/brushing-and-flossing/safe-storage-for-family-toothbrushes-0413. Accessed May 13, 2021.
- Konidala U, Nuvvula S, Mohapatra A, Nirmala SV. Efficacy of various disinfectants on microbially contaminated toothbrushes due to brushing. Contemp Clin Dent 2011; 2(4):302-7. doi: 10.4103/0976-237x.91793 [Crossref] [ Google Scholar]
- Naik R, Ahmed Mujib BR, Telagi N, Anil BS, Spoorthi BR. Contaminated tooth brushes-potential threat to oral and general health. J Family Med Prim Care 2015; 4(3):444-8. doi: 10.4103/2249-4863.161350 [Crossref] [ Google Scholar]
- Sato S, Ito IY, Lara EH, Panzeri H, Albuquerque Junior RF, Pedrazzi V. Bacterial survival rate on toothbrushes and their decontamination with antimicrobial solutions. J Appl Oral Sci 2004; 12(2):99-103. doi: 10.1590/s1678-77572004000200003 [Crossref] [ Google Scholar]
- Lamarca JH, de Carvalho FG, Machado FC, Lacerda-Santos R, Barbosa TS. Severe acute respiratory syndrome coronavirus 2: a protocol for disinfection of toothbrushes. J Infect Dis 2021; 223(6):1113-4. doi: 10.1093/infdis/jiaa794 [Crossref] [ Google Scholar]
- Türkoğlu O, Becerik S, Emingil G, Kütükçüler N, Baylas H, Atilla G. The effect of adjunctive chlorhexidine mouthrinse on clinical parameters and gingival crevicular fluid cytokine levels in untreated plaque-associated gingivitis. Inflamm Res 2009; 58(5):277-83. doi: 10.1007/s00011-008-8129-z [Crossref] [ Google Scholar]
- Amtha R, Kanagalingam J. Povidone-iodine in dental and oral health: a narrative review. J Int Oral Health 2020; 12(5):407-12. doi: 10.4103/jioh.jioh_89_20 [Crossref] [ Google Scholar]
- Muniz F, Cavagni J, Langa GPJ, Stewart B, Malheiros Z, Rösing CK. A systematic review of the effect of oral rinsing with H2O2 on clinical and microbiological parameters related to plaque, gingivitis, and microbes. Int J Dent 2020; 2020:8841722. doi: 10.1155/2020/8841722 [Crossref] [ Google Scholar]
- Stathis C, Victoria N, Loomis K, Nguyen SA, Eggers M, Septimus E. Review of the use of nasal and oral antiseptics during a global pandemic. Future Microbiol 2021; 16(2):119-30. doi: 10.2217/fmb-2020-0286 [Crossref] [ Google Scholar]
- Mali AM, Behal R, Gilda SS. Comparative evaluation of 01% turmeric mouthwash with 02% chlorhexidine gluconate in prevention of plaque and gingivitis: a clinical and microbiological study. J Indian Soc Periodontol 2012; 16(3):386-91. doi: 10.4103/0972-124x.100917 [Crossref] [ Google Scholar]
- Waghmare PF, Chaudhari AU, Karhadkar VM, Jamkhande AS. Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: a clinical and microbiological study. J Contemp Dent Pract 2011; 12(4):221-4. doi: 10.5005/jp-journals-10024-1038 [Crossref] [ Google Scholar]
- Arunachalam LT, Sudhakar U, Vasanth J, Khumukchum S, Selvam VV. Comparison of anti-plaque and anti-gingivitis effect of curcumin and chlorhexidine mouth rinse in the treatment of gingivitis: a clinical and biochemical study. J Indian Soc Periodontol 2017; 21(6):478-83. doi: 10.4103/jisp.jisp_116_17 [Crossref] [ Google Scholar]
- Abdulrab S, Alkadasi B, Al-Maweri S, Halboub E, Alhadainy H, Geerts G. Statins-based prophylactic mouthwash and nasal spray may protect against coronavirus disease 2019. New Microbes New Infect 2020; 37:100751. doi: 10.1016/j.nmni.2020.100751 [Crossref] [ Google Scholar]
- Al Habashneh R, Qubain TG, Alsalman W, Khader Y. The effect of listerine mouthwash on dental plaque, gingival inflammation and C-reactive protein (CRP). Dentistry 2014; 4(2):191. doi: 10.4172/2161-1122.1000191 [Crossref] [ Google Scholar]
- Alshehri FA. The use of mouthwash containing essential oils (LISTERINE®) to improve oral health: a systematic review. Saudi Dent J 2018; 30(1):2-6. doi: 10.1016/j.sdentj.2017.12.004 [Crossref] [ Google Scholar]
- Parashar A. Mouthwashes and their use in different oral conditions. Sch J Dent Sci 2015; 2(2B):186-91. [ Google Scholar]
- Sharma S, Saimbi CS, Koirala B, Shukla R. Effect of various mouthwashes on the levels of interleukin-2 and interferon-gamma in chronic gingivitis. J Clin Pediatr Dent 2008; 32(2):111-4. doi: 10.17796/jcpd.32.2.u01p135561161476 [Crossref] [ Google Scholar]
- Chatterjee A, Debnath K, Rao NKH. A comparative evaluation of the efficacy of curcumin and chlorhexidine mouthrinses on clinical inflammatory parameters of gingivitis: a double-blinded randomized controlled clinical study. J Indian Soc Periodontol 2017; 21(2):132-7. doi: 10.4103/jisp.jisp_136_17 [Crossref] [ Google Scholar]
- Brookes ZLS, Bescos R, Belfield LA, Ali K, Roberts A. Current uses of chlorhexidine for management of oral disease: a narrative review. J Dent 2020; 103:103497. doi: 10.1016/j.jdent.2020.103497 [Crossref] [ Google Scholar]
- Kolahi J, Soolari A. Rinsing with chlorhexidine gluconate solution after brushing and flossing teeth: a systematic review of effectiveness. Quintessence Int 2006; 37(8):605-12. [ Google Scholar]
- FDI Commission. Mouthrinses and periodontal disease. Int Dent J 2002; 52(5):346-52. [ Google Scholar]
- DePaola LG, Spolarich AE. Safety and efficacy of antimicrobial mouthrinses in clinical practice. J Dent Hyg 2007; 81(5):1-16. [ Google Scholar]
- PERIDEX - chlorhexidine gluconate mouthwash. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019028s020lbl.pdf. Accessed May 2, 2021.
- Soumya P, Mohanraj S, Manipal S, Prabu D, Bharathwaj VV, Rajmohan M. Effects of chlorhexidine on taste perception: a systematic review. J Pharm Sci Res 2019; 11(10):3468-74. [ Google Scholar]
- O’Donnell VB, Thomas D, Stanton R, Maillard JY, Murphy RC, Jones SA. Potential Role of Oral Rinses Targeting the Viral Lipid Envelope in SARS-CoV-2 Infection. Function 2020; 1(1):zqaa002. doi: 10.1093/function/zqaa002 [Crossref] [ Google Scholar]
- World Health Organization Model List of Essential Medicines, 21st List. 2019. https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06.
- Rosling B, Hellström MK, Ramberg P, Socransky SS, Lindhe J. The use of PVP-iodine as an adjunct to non-surgical treatment of chronic periodontitis. J Clin Periodontol 2001; 28(11):1023-31. doi: 10.1034/j.1600-051x.2001.281106.x [Crossref] [ Google Scholar]
- Frank S, Capriotti J, Brown SM, Tessema B. Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era. Ear Nose Throat J 2020; 99(9):586-93. doi: 10.1177/0145561320932318 [Crossref] [ Google Scholar]
- Vergara-Buenaventura A, Castro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg 2020; 58(8):924-7. doi: 10.1016/j.bjoms.2020.08.016 [Crossref] [ Google Scholar]
- Rashed HT. Evaluation of the effect of hydrogen peroxide as a mouthwash in comparison with chlorhexidine in chronic periodontitis patients: a clinical study. J Int Soc Prev Community Dent 2016; 6(3):206-12. doi: 10.4103/2231-0762.183114 [Crossref] [ Google Scholar]
- Mathurasai W, Thanyasrisung P, Sooampon S, Ayuthaya BIN. Hydrogen peroxide masks the bitterness of chlorhexidine mouthwash without affecting its antibacterial activity. J Indian Soc Periodontol 2019; 23(2):119-23. doi: 10.4103/jisp.jisp_414_18 [Crossref] [ Google Scholar]
- Prabhu R, Kohale B, Agrawal AA, Wagle SV, Bhartiya G, Chaudhari D. A comparative clinical study to evaluate the effect of 15% hydrogen peroxide mouthwash as an adjunct to 02% chlorhexidine mouthwash to reduce dental stains and plaque formation. Int J Contemp Med Res 2017; 4(10):2181-4. [ Google Scholar]
- Livada R, Shiloah J, Tipton DA, Dabbous MK. The potential role of curcumin in periodontal therapy: a review of the literature. J Int Acad Periodontol 2017; 19(3):70-9. [ Google Scholar]
- Nosratzehi T, Nosratzehi M, Nosratzehi S, Lotfi F. The comparison of the effect of curcumin with nystatin on inhibition level of Candida albicans. J Exp Pharmacol 2019; 11:93-7. doi: 10.2147/jep.s215843 [Crossref] [ Google Scholar]
- Murugesh J, Annigeri RG, Mangala GK, Mythily PH, Chandrakala J. Evaluation of the antifungal efficacy of different concentrations of Curcuma longa on Candida albicans: an in vitro study. J Oral Maxillofac Pathol 2019; 23(2):305. doi: 10.4103/jomfp.JOMFP_200_18 [Crossref] [ Google Scholar]
- Abdi Z, Roozegar MA, Beigi S, Khorshidi A, Azizian M, Beigi Z. Using simvastatin mouthwash (06%, 12% and 18%) on the improvement of clinical parameters in patients with chronic periodontitis. Res J Pharm Technol 2020; 13(2):862-6. doi: 10.5958/0974-360x.2020.00163.8 [Crossref] [ Google Scholar]
- Roozegar MA, Abdi Z, Matin S, Khorshidi A, Azizian M. The effect of 12 mg simvastatin mouthwash on the level of interleukin-6 in patients with chronic periodontitis. Indian J Forensic Med Toxicol 2019; 13(3):463-7. [ Google Scholar]
- Hasan F, Ikram R, Simjee SU, Iftakhar K, Asadullah K. Effectiveness of simvastatin 1% oral gel and mouthwash used as an adjunct treatment of scaling and root planning in the treatment of periodontal diseases. Pak J Pharm Sci 2019; 32(6):2673-7. [ Google Scholar]
- Pradeep Kumar S, Athiban Raj J. Effects of alcohol containing mouthwash on oral tissue: a review. Int J Sci Res 2017; 6(6):1584-7. [ Google Scholar]
- Pradeep AR, Kumari M, Priyanka N, Naik SB. Efficacy of chlorhexidine, metronidazole and combination gel in the treatment of gingivitis--a randomized clinical trial. J Int Acad Periodontol 2012; 14(4):91-6. [ Google Scholar]
- Mishra A, Harshitha B, Reddy K. Comparative evaluation of adjunctive use of four commercially available antimicrobial topical gels in chronic gingivitis: a clinical study. J Appl Dent Med Sci 2015; 1(3):19-25. [ Google Scholar]