Avicenna J Dent Res. 14(2):74-79.
doi: 10.34172/ajdr.2022.17
Original Article
A Comparison of the Expression of Cyclin D1 in OSCC and CSCC
Shirin Modabbernia 1, *  , Hadise Mousapour 2, Parisa Rahimirad 3, Shima Daryoush 3
, Hadise Mousapour 2, Parisa Rahimirad 3, Shima Daryoush 3 
Author information:
1DDS, Pathologist, Department of Oral and Maxillofacial Pathology, Guilan University of Medical Sciences, Rasht, Iran
2Student of Dentistry, Guilan University of Medical Sciences, Rasht, Iran
3Student Research Committee, School of Dentistry, Guilan University of Medical Sciences, Rasht, Iran
*
Corresponding author: Shirin Modabbernia, Faculty of Dentistry, Dental School, Guilan University of Medical Sciences, Rasht, Iran, Tel: +98- 9111361593, Email:
shirinmodabbernia@yahoo.com
Abstract
Background: There are several types of carcinoma which may cause serious diseases in humans and lead to their death. Neoplasia in squamous cells is a subtype of carcinoma which can cause squamous cell carcinoma (SCC). SCC can occur in several areas in the oral cavity (oral squamous cell carcinoma, OSCC) and cutaneous tissues (cutaneous squamous cell carcinoma, CSCC) such as skin. This study aims to investigate the expression level of cyclin D1 and its relevance to different prognoses of OSCC and CSCC. The present study investigates the expression of cyclin D1 and its relevance to different prognoses of OSCC and CSCC. Indexes such as lesion site, gender, and age have been checked.
Methods: In this cross-sectional descriptive-analytical study, 23 cases of OSCC and 23 cases of CSCC were evaluated. The immunohistochemistry (IHC) staining method was employed to study the correlation of cyclin D1 and the above-mentioned SCCs. The data were analyzed using KAI2, Fisher’s exact test, Mann-Whitney U test, and dependent t tests in SPSS version 22.0. Statistical significance was set at P<0.05.
Results: The results showed that staining status was not significantly correlated with lesion site (P=0.999). According to the results, there was no significant relationship between staining pattern and lesion site (P=0.749). There was a significant relationship between the severity of staining and lesion site (P=0.040). In addition, those with skin lesions showed higher staining intensity. The staining status of gender or age groups was not affected by the adjustment of lesion site. By moderating the effect of lesion site and age group, gender was found to affect staining pattern (P=0.036). The odds ratio of having a diffuse pattern was 4.90 times higher in men than in women. Regardless of the independent variables in the model of people with color intensity 2, their likelihood of going to color 4 was significantly higher (P<0.001). People in intensity 0 were significantly less likely to go to 4 (P=0.001). People in intensity 1 had no significant relationship with those in intensity 4 (P=0.405). Men were less likely to go higher than women. Furthermore, people aged 72 and under were less likely to go higher. Individuals with skin lesions were more likely to go to higher intensity, even though none of the independent variables was significant.
Conclusions: The staining intensity was higher in CSCC than in OSCC. A lot of factors are associated with the prognosis of SCCs, and cyclin D1 may be used as a prognostic marker.
Keywords: Cyclin D1, Squamous cell carcinoma, Prognosis, Skin, Immunohistochemistry
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Modabbernia S, Mousapour Siahkesh H, Rahimirad P, Daryoush S. A comparison of the expression of cyclin d1 in oscc and cscc. Avicenna J Dent Res. 2022; 14(2):74-79. doi:10.34172/ajdr.2022.17
Introduction
Squamous cell carcinoma (SCC) consists of several subtypes. This kind of cancer develops due to an uncontrolled growth of abnormal squamous cells (1). Long-term exposure to ultraviolet (UV) radiation causes most SCCs (2). In Iran, 5.2%-37.2% of all cancers are diagnosed as CSCC, which is one of the most common kinds of cancers in the world (3). The studies conducted between 1962 and 2009 in academic centers and hospitals demonstrated that the prevalence of oral squamous cell carcinoma (OSCC) in Tehran, Mashhad, and Shiraz was 1.29%-1.6%, 0.9%-3.1%, and 1.4%-5%, respectively (4). Tobacco exposure, alcohol dependence, and infections with oncogenic viruses which affect the cell cycle can be considered as the risk factors for developing oral cancers (1). Cyclin D1 is a protein encoded in the ccD1 sequence (PRAD-1 or BC-1) of chromosome 13q11 (5) and an oncogene involved in cell cycle regulation (6). During cell cycle, cyclin D1 attaches to cyclin-dependent kinase 4 or 6 and promotes progression of the cell cycle from G1 to S phase by phosphorylation of protein retinoblastoma (7). As the expression of cyclin D1 reduced, SCC cells were induced G0/G1 arrest and cell division was substantially blocked (8). SSC may be formed by the changes in oncogenes and tumor suppressor genes such as p53 or p16 (9). For example, overexpression of cyclin D1 can increase cancer aggressiveness by desensitizing cell proliferation to inhibitory signals (10). It shortens G1 phase and leads to less dependency on growth factors, hence the abnormal cell proliferation (11). Unlike cutaneous squamous cell carcinoma (CSCC), the prognosis of OSCC is poor and its five-year survival rate is less than 50% (12). In fact, a lot of factors affect the prognosis, one of which can be cyclin D1. Compared to other OSCCs, the OSCCs with overexpression of cyclin D1 have poorer prognosis (13). On the contrary, in the study conducted by Ahmed Haji Omar, OSCC demonstrated poorer prognosis than CSCCS (14). Therefore, the present article investigated the effect of cyclin D1 on poor prognosis of OSCC and its difference in CSCC and OSCC. The study aimed to predict the prognosis and choose the best treatment for decreasing the consequences of these carcinomas.
Materials and Methods
The present cross-sectional descriptive-analytical study was performed on paraffin-embedded tissue blocks of oral and skin SCCs available in laboratories of Rasht in 2020. Sampling was done by examining the available samples, reviewing the available slides, and selecting appropriate samples. First, 23 cases of OSCC and 23 cases of CSCC were randomly selected and the expression status of cyclin D1 in these two types of SCC was measured and compared.
The samples with SCC being re-diagnosed by a pathologist, sufficient tissue for investigation, fully recorded radiology findings, and demographic information were included in the study. The samples with SCC not being re-diagnosed by a pathologist, insufficient tissue for investigation, unavailable blocks or incomplete information were excluded from the study.
Immunohistochemistry (IHC) staining is a method of detecting certain protein markers using antibodies (15). Prepared mouse anti-cyclin D1 monoclonal antibody (Demark dako, ready to use, N16187) was used together with peroxidase antiperoxidase method. The material kit was prepared by the master company, the required brochures were painted, and the slides were stained by using the EnVision method.
In this study, 4-μm-thick sections were cut from every specimen and placed on microscope slides. To remove the paraffin, the slides were put in the oven at 58°C for 24 hours. They were subsequently placed in 2 containers of xylene for 5 minutes. Then alcohol with different grades (70% to 100%) was used for hydration. After being washed with distilled water, the slides were placed in citrate buffer solution (pH = 9) for antigen retrieval. The solution was first placed in the microwave for 5 minutes with the power of 80 W and then for 15 minutes with the power of 450 W. After the slides were washed (for 15 minutes) and dried out, to inhibit internal peroxidase, all the specimens were incubated in hydrogen peroxide 3% for 20 minutes. To inhibit internal peroxidase, all the specimens were incubated in hydrogen peroxide 3% for 20 minutes after the slides were washed (for 15 minutes) and dried out. Following this step, the specimens were washed with phosphate buffered saline. This process was repeated amid adding primary and secondary antibody, diaminobenzidine chromogen, and hematoxylin for coloring. In the final step, the specimens were placed in different grades of alcohol to become dehydrated. They were subsequently placed in xylene to become clear.
Slides were first observed using low magnification and the areas with maximum immunoreactivity were determined. Then, at 400× magnitude,1000 epithelial cells were counted in these areas, and the percentage of stained cells, Label Index: LI, was calculated for each sample. Light microscopy was used to examine each specimen, and cells with brown nuclei were counted as stained cells. Olympus Binocular microscope (CX23, made in Japan) was used in this study.
If the percentage of stained cells is less than 1%, it is considered 0, and 1-10% is known as +1, 10-35% is known as +2, 35-70% is known as +3, and percentages higher than 70% are considered +4 (16).
The data were analyzed using one-way analysis of variance (ANOVA) and Tukey tests (for comparing quantitative variables between groups), Kruskal-Wallis and Mann-Whitney tests (for comparing semi-quantitative variables between groups), and χ2 and Fisher’s Exact test (for comparing qualitative variables) in SPSS version 22.0. Intergroup comparisons were also made. A significance level of less than 0.05 was considered in all tests.
Results
The present study included 29 male and 17 female participants. Based on the results, 44.8% of male participants had oral and 55.2% had skin lesions. The rates of oral and skin lesions for female participants were 58.8% and 41.2%, respectively. The mean age of the participants with oral lesions was 66.22 ± 15.25. This mean was 74.48 ± 14.49 for the ones who had skin lesions.
Table 1 presents some information about the participants’ lesion site, gender, and age. The mean ages of patients with skin and oral lesions were 74.48 ± 14.49 and 66.22 ± 15.25 years. The first objective of the study was analyzed by Fisher’s exact test.
Table 1.
Determination of Gender and Age Group of Participants in the Study Based on Lesion Site
|
Variable
|
Category
|
Lesion Site
|
Statistics
|
P
Value
|
|
|
Oral (%)
|
Skin (%)
|
| Gender |
Male |
13 (44.8) |
16 (55.2) |
0.84 |
0.395 |
| Female |
10 (58.8) |
7 (41.2) |
| Age |
≥72 |
13 (59.1) |
9 (40.9) |
1.39 |
0.283 |
| < 72 |
10 (41.7) |
14 (58.3) |
Determining and Comparing the Staining Intensity of Cyclin D1 in OSCC
Fisher’s exact test was used to analyze the first objective of the study. The results showed that staining status was not significantly correlated with lesion site (P = 0.999) (Table 2)
Table 2.
Comparison of Staining Intensity of Cyclin D1 in Oral Squamous Cell Carcinoma and Staining Pattern of Cyclin D1 in Oral and Skin Squamous Cell Carcinoma
|
Staining Pattern Cyclin D1
|
Staining Intensity of Cyclin D1
|
The Location of Lesion (%)
|
Statistics
|
P
Value
|
|
Oral
|
Skin
|
| - |
Negative |
1 (50) |
1 (50) |
- |
0.999 |
| Positive |
22 (50) |
22 (50) |
| Focal |
- |
5 (38.5) |
8 (61.5) |
1.21 |
0.749 |
| Releasing |
17 (54.8) |
14 (45.2) |
| - |
1 (50) |
1 (50) |
Determining and Comparing the Staining Pattern of Cyclin D1 in Oral Squamous Cell Carcinoma
The results of Fisher’s exact test, which was used to analyze the second objective of the study, are presented in Table 2. According to the results, there was no significant relationship between staining status and lesion site (P = 0.749).
Determining and Comparing Cyclin D1 Staining Intensity in OSCC
Chi-square test was used to analyze the second objective of the study. According to the results, there was a significant relationship between the severity of staining and lesion site (P = 0.040), and those with skin lesions had higher staining intensity (Table 3 and Figure 1).
Table 3.
Comparison of Staining Intensity of Cyclin D1 in Oral and Skin Squamous Cell Carcinoma
|
Location of Lesion
|
Staining Intensity of Cyclin D1
|
Statistics
|
P Value |
|
0 (<%1) No. (%)
|
+1 (%1- %10) No. (%)
|
+2 (%10- %35) No. (%)
|
+4 (>%70) No. (%)
|
| Skin |
1 (4.3) |
10 (43.5) |
9 (39.1) |
3 (13) |
4.21 |
0.040 |
| Oral |
1 (4.3) |
16 (69.6) |
6 (26.1) |
0 (0) |
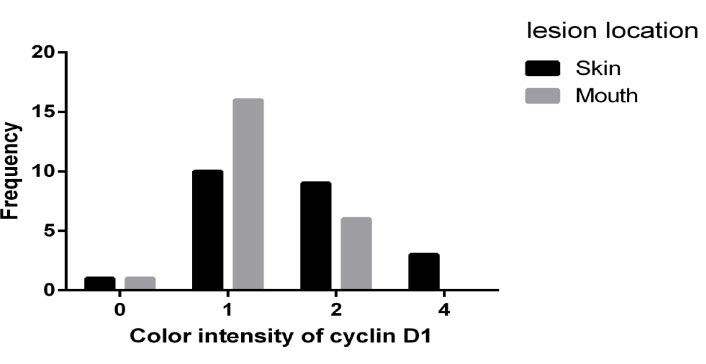
Figure 1.
Frequency of Staining Intensity Based on Lesion Site
.
Frequency of Staining Intensity Based on Lesion Site
Comparing the Staining Intensity of Cyclin D1 in Oral and Skin Squamous Cell Carcinoma Based on Gender and Age
Binary logistic regression was used to analyze the fourth objective of the study.
According to the results obtained by modifying lesion site, neither gender nor age had any effect on staining status (Table 4)
Table 4.
Comparison of Staining Intensity and Staining Pattern of Cyclin D1 in Oral and Skin Squamous Cell Carcinoma Based on Gender and Age
|
Variable
|
Category
|
OR
|
95% CI
|
P
Value
|
|
Cyclin D1 Staining Pattern
|
Cyclin D1 Staining
|
Cyclin D1 Staining Pattern
|
Cyclin D1 Staining
|
Cyclin D1 Staining Pattern
|
Cyclin D1 Staining
|
| Gender |
Female |
1 |
1 |
1.11-21.62 |
- |
0.036 |
- |
| Male |
4.90 |
0 |
| Age category |
≥72 |
1 |
1 |
0.09-1.76 |
0.06 – 18.93 |
0.223 |
0.972 |
| < 72 |
0.39 |
1.05 |
| Location of lesion |
Skin |
1 |
1 |
0.49-10.05 |
0.04 – 14.45 |
0.303 |
0.882 |
| Oral |
2.21 |
0.80 |
Comparing the Staining Pattern of Cyclin D1 in Oral and Skin Squamous Cell Carcinoma Based on Gender and Age
A binary logistic regression test was used to analyze the fifth objective of the study. According to the results obtained by moderating the effect of lesion site and age, gender had an effect on staining pattern (P = 0.036). The odds ratio of having a diffuse pattern was 4.90 times higher in men than in women (Table 4, Figure 2).
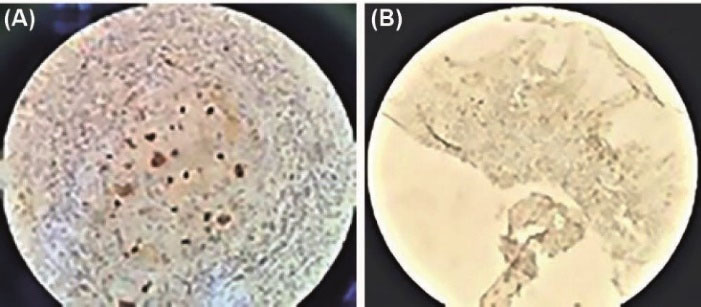
Figure 2.
(A) Immunohistochemical Analysis Reveals Cyclin D1 Focal/Diffused Expression Pattern in SCC, ×40. (B). Immunohistochemical Analysis Reveals Cyclin D1 Release/Diffusion Expression Pattern in SCC, ×10
.
(A) Immunohistochemical Analysis Reveals Cyclin D1 Focal/Diffused Expression Pattern in SCC, ×40. (B). Immunohistochemical Analysis Reveals Cyclin D1 Release/Diffusion Expression Pattern in SCC, ×10
Comparing the Staining Intensity of Cyclin D1 in Oral and Skin Squamous Cell Carcinoma Based on Gender and Age
Sequential regression analysis was used to analyze the sixth objective of the study. Regardless of the independent variables in the model of people with color intensity 2, the likelihood of going to color 4 was significantly higher (P < 0.001). People in intensity 0 were significantly less likely to go to 4 (P = 0.001). People in intensity 1 had no significant relationship with intensity 4 (P = 0.405). Other results showed that men were significantly less likely to go higher than women. In addition, people aged 72 and under were less likely to go higher.
Individuals with skin lesions were more likely to go to extremes, even though none of the independent variables was significant (Table 5 and Figure 3).
Table 5.
Comparison of Staining Intensity of Cyclin D1 in Oral and Skin Squamous Cell Carcinoma Based on Gender and Age
|
Role
|
Variable
|
Category
|
Estimate
|
Statics
|
P
Value
|
| Response |
Intensity of staining |
0 |
-3.21 |
12.07 |
0.001 |
| 1 |
0.54 |
0.69 |
0.405 |
| 2 |
2.89 |
12.13 |
> 0.001 |
| 4 |
Reference |
| Predictor |
Gender |
Male |
-0.39 |
0.41 |
0.524 |
| Female |
Reference |
| Predictor |
Age category |
≥72 |
-0.47 |
0.60 |
0.440 |
| < 72 |
Reference |
| Predictor |
Location of lesion |
Skin |
1.07 |
2.69 |
0.085 |
| Oral |
Reference |
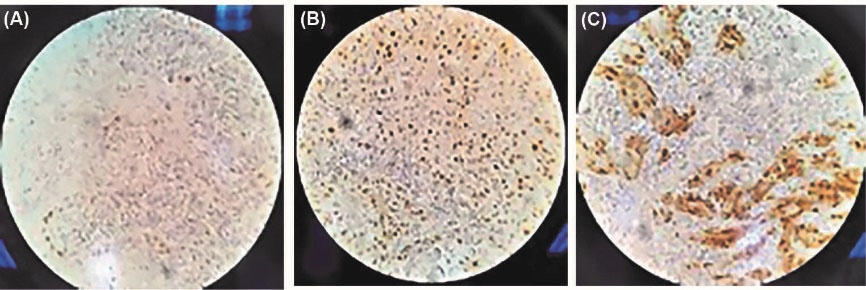
Figure 3.
(A) Immunohistochemical analysis shows +1 intensity staining of cyclin D1 expression, ×40. (B). Immunohistochemical analysis shows +2 intensity staining of cyclin D1 expression, ×40. (C). Immunohistochemical analysis shows +4 intensity staining of cyclin D1 expression, ×40.
.
(A) Immunohistochemical analysis shows +1 intensity staining of cyclin D1 expression, ×40. (B). Immunohistochemical analysis shows +2 intensity staining of cyclin D1 expression, ×40. (C). Immunohistochemical analysis shows +4 intensity staining of cyclin D1 expression, ×40.
Discussion
The present study was conducted to investigate the immunohistochemical reactivity and association of lesion site with staining pattern. In addition, it aimed to analyze the relationship between “the staining intensity of cyclin D1 in OSCC and CSCC” and “participants’ age and gender”.
Changes in the factors controlling the cell cycle may lead to SCCs. For instance, the overexpression of cyclin D1 was observed in most of the participants with SCC (99% of both OSCC and CSCC specimens)(6). Normal skin was negative for cyclin D1 immunostaining. Shen et al reported that in most of the CSCC specimens, cyclin D1 was found to be positive. A total of 54 patients were collected from Huashan Hospital over 3 years. Additionally, 24 CSCC and 8 normal individuals served as the control group. The IHC scoring results for cyclin D1 were based on staining intensity and frequency distribution of immunopositive cells. The difference between the control and cancer groups was statistically significant (P < 0.05). However, there was no correlation between the expression of cyclin D1 and SCC grading (17).
The study conducted by John et al included 20 cases of OSCC and 20 healthy individuals. The OSCC group consisted of 8 females and 12 males in the age group of 35–79 years, with a mean age of 59 ± 11.48. The healthy individual group consisted of 8 females and 12 males in the age group of 26-86 years, with a mean age of 52.7 ± 18.1. The IHC was done by using polymer-labelling technique (Dako EnVision). There was no statistically significant difference between males and females in the cyclin D1 score (P = 0.05) and the cyclin D1 score did not show a statistically significant difference with histopathological diagnosis of OSCC (P = 0.05) (18). In the present study, there was no correlation between the expression of cyclin D1 and lesion site. The cyclin D1 score did not show a significant difference (P = 0.337) among the different histopathological diagnoses of OSCC. No statistically significant association was found between cyclin D1 score and the prognosis of the disease among the OSCC patients (P = 0.239). Since the overexpression of cyclin D1 mainly affects neoplastic transformation rather than differentiation of tumor cells, it can be a good marker for the prognosis of SCC in the early stage (18).
In the present study, there was no correlation between the expression of cyclin D1 and lesion site. John et al examined specimens from different parts of the mouth such as maxilla, tongue, buccal mucosa, and mandibular alveolus and found no correlation between the expression of cyclin D1 and lesion site (18). The present researchers realized that there was no correlation between staining pattern and lesion site (P = 0.749).
A cross-sectional study was conducted by Dodani et al on 40 cases of OSCC and CSCC and 20 cases of normal skin and normal oral mucosa. They found no significant differences in the presence and accumulation of myofibroblasts in OSCC and CSCC in terms of the intensity and pattern of staining. Using toluidine blue staining, they showed that the number of mast cells was higher in CSCC than in OSCC (19). A retrospective study was performed on 64 anatomopathological reports following the surgical removal of squamous cell carcinomas. Unlike the staining pattern, the present researchers found a correlation between lesion site and the staining intensity of cyclin D1. As a result, skin lesion showed a more intense response.
Using toluidine blue staining, Kadeh and Saravani showed that the number of mast cells was higher in CSCC than in OSCC. This study was conducted on 60 samples including 30 OSCC and 30 CSCC (12).
The differences in the results of the two studies mentioned above and the present research can be due to the use of different methods and samples.
Guan et al examined 10 formalin-fixed, paraffin-embedded oral mucosal biopsies with the diagnosis of non-neoplastic and non-dysplastic tissues, 12 samples of mild to moderate oral epithelial dysplasia, and 11 samples of OSCC for the period from 2005 to 2013. Using IHC, they detected a higher level of the expression of cyclin D1 in OSCC compared to oral epithelial dysplasia (P = 4 X 10-5 ). There were no statistically significant differences among these three groups. A higher expression of cyclin D1 was detected in moderately differentiated OSCC when compared with well-differentiated OSCC (P = 0.007). OSCC specimens showed the highest score of nuclear and cytoplasmic staining. The relationship between the expression of cyclin D1 and prognosis in OSCC seemed to be complicated (20).
In addition, the relationship of age and gender with the above-mentioned parameters was investigated. By modifying lesion site, there was a correlation between gender and staining pattern and the diffuse pattern was 4 times higher in men than in women.
Furthermore, the present article investigated the relationship of the staining intensity of cyclin D1 with gender and age. It was observed that men were significantly less likely to go higher than women. In addition, people aged 72 and under were less likely to go to higher intensity. However, no statistically significant difference was found. It has to be noted that lack of enough specimens may have affected the result.
Servani et al conducted a study on 16 OLP patients, with 16 participants as the control group. IHC for Ki-67 was conducted by using the EnVision method. It was reported that the number of mast cells stained with toluidine blue had no relationship with age or gender (12).
Sadri et al reported that the expression of antigen ki67 was higher in esophagus SCC than in oral SCC. It was also realized that ki67 would be a good marker for prognosis (21).
In a study conducted by Ahmed Haji Omar et al, 36 OSCC and 27 CSCC samples were examined. In their study, OSCC showed a poorer prognosis than CSCC. Multiple differences were found between these tumors, which were situated in the tumor cells, the tumor stromal cells, the communication between tumor cells and stromal cells, and the communication within tumor cells or stromal cells (14).
The relationship between the expression of cyclin D1 and prognosis in OSCC seemed to be complicated (22).HPV-related OSCC was reported to be strongly associated with the expression of low cyclin D1 and the majority of HPV-negative tumors showed the overexpression of cyclin D1 (23).
It was found that the overexpression of cyclin D1 was a powerful predictor of adverse outcomes in OSCCs (24).
Conclusions
The expression of cyclin D1 is higher in skin squamous cell carcinoma than in the oral cavity. Therefore, the cyclin D1 marker may be used to improve the prognosis of lesions.
Acknowledgements
All authors have made substantive contributions to this study and all have reviewed the final version of the manuscript prior to its submission.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Ethical Statement
This study was approved by National Research Ethics Committee (No. 98283).
References
- Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc 2016; 91(3):386-96. doi: 10.1016/j.mayocp.2015.12.017 [Crossref] [ Google Scholar]
- Saraiya M, Glanz K, Briss PA, Nichols P, White C, Das D. Interventions to prevent skin cancer by reducing exposure to ultraviolet radiation: a systematic review. Am J Prev Med 2004; 27(5):422-66. doi: 10.1016/j.amepre.2004.08.009 [Crossref] [ Google Scholar]
- Ghoncheh M, Koohi F, Salehiniya H. Epidemiology and trend of skin cancer incidence in southern Iran. J Dermatol Cosmet 2015;6(2):85-92. [Persian].
- Ghafari R, Jalayer Naderi N, Emami Razavi A. A retrospective institutional study of histopathologic pattern of oral squamous cell carcinoma (OSCC) in Tehran, Iran during 2006-2015. J Res Med Sci 2019; 24:53. doi: 10.4103/jrms.JRMS_882_18 [Crossref] [ Google Scholar]
- John RR, Malathi N, Ravindran C, Anandan S. Mini review: multifaceted role played by cyclin D1 in tumor behavior. Indian J Dent Res 2017; 28(2):187-92. doi: 10.4103/ijdr.IJDR_697_16 [Crossref] [ Google Scholar]
- Caulin C, Nguyen T, Lang GA, Goepfert TM, Brinkley BR, Cai WW. An inducible mouse model for skin cancer reveals distinct roles for gain- and loss-of-function p53 mutations. J Clin Invest 2007; 117(7):1893-901. doi: 10.1172/jci31721 [Crossref] [ Google Scholar]
- Ma HB, Hu HT, Di ZL, Wang ZR, Shi JS, Wang XJ. Association of cyclin D1, p16 and retinoblastoma protein expressions with prognosis and metastasis of gallbladder carcinoma. World J Gastroenterol 2005; 11(5):744-7. doi: 10.3748/wjg.v11.i5.744 [Crossref] [ Google Scholar]
- Ye L, Zhang J, Zhang Y, Gu B, Zhu H, Mao X. Isoliquiritigenin suppressed esophageal squamous carcinoma growth by blocking EGFR activation and inducing cell cycle arrest. Biomed Res Int 2020; 2020:9259852. doi: 10.1155/2020/9259852 [Crossref] [ Google Scholar]
- Rivera C, Venegas B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncol Lett 2014; 8(1):7-11. doi: 10.3892/ol.2014.2103 [Crossref] [ Google Scholar]
- Jadhav KB, Gupta N. Clinicopathological prognostic implicators of oral squamous cell carcinoma: need to understand and revise. N Am J Med Sci 2013; 5(12):671-9. doi: 10.4103/1947-2714.123239 [Crossref] [ Google Scholar]
- Todd R, Hinds PW, Munger K, Rustgi AK, Opitz OG, Suliman Y. Cell cycle dysregulation in oral cancer. Crit Rev Oral Biol Med 2002; 13(1):51-61. doi: 10.1177/154411130201300106 [Crossref] [ Google Scholar]
- Kadeh H, Saravani S. A comparative study of the mast cells count between oral squamous cell carcinoma and cutaneous squamous cell carcinoma. J Mazandaran Univ Med Sci 2016;26(142):60-7. [Persian].
- Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res 2008; 87(1):14-32. doi: 10.1177/154405910808700104 [Crossref] [ Google Scholar]
- Ahmed Haji Omar A. Oral and Cutaneous Squamous Cell Carcinomas: Differences Between Tumors and Their Microenvironments [dissertation]. Helsinki: University of Helsinki, 2015. p. 123.
- Ramos-Vara JA, Miller MA. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet Pathol 2014; 51(1):42-87. doi: 10.1177/0300985813505879 [Crossref] [ Google Scholar]
- Zargaran M, Jamshidi S, Baradaran A, Moghimbeigi A, Alikhassi M. Comparative investigation of cyclin D1 expression in oral lichen planus and squamous cell carcinoma by immunohistochemistery technique. J Mashhad Dent Sch 2014; 38(1):17-28. doi: 10.22038/jmds.2014.2135 [Crossref] [ Google Scholar]
- Shen Y, Xu J, Jin J, Tang H, Liang J. Cyclin D1 expression in Bowen’s disease and cutaneous squamous cell carcinoma. Mol Clin Oncol 2014; 2(4):545-8. doi: 10.3892/mco.2014.273 [Crossref] [ Google Scholar]
- John RR, Ravindran C, Malathi N, Aruna RM. Evaluation of the role played by cyclin D1 as a diagnostic and prognostic marker in the progression of oral carcinogenesis. J Maxillofac Oral Surg 2018; 17(3):389-95. doi: 10.1007/s12663-018-1087-2 [Crossref] [ Google Scholar]
- Dodani A, Siadati S, Salehinejad J, Hajian-Tilaki K, Abbaszadeh-Bidokhty H. Comparative evaluation of the frequency of myofibroblasts between oral and cutaneous squamous cell carcinomas. Caspian J Dent Res 2016; 5(2):24-9. doi: 10.22088/cjdr.5.2.24 [Crossref] [ Google Scholar]
- Guan G, Bakr MM, Firth N, Love RM. Expression of cyclin D1 correlates with p27KIP1 and regulates the degree of oral dysplasia and squamous cell carcinoma differentiation. Oral Surg Oral Med Oral Pathol Oral Radiol 2018; 126(2):174-83. doi: 10.1016/j.oooo.2018.01.015 [Crossref] [ Google Scholar]
- Sadri d, Shahsavari F, Alavi S. Expresion of Ki67antigen in oral and esophagus squamous cell carcinoma. J Res Dent Sci 2011;8(1):34-40. [Persian].
- Perisanidis C, Perisanidis B, Wrba F, Brandstetter A, El Gazzar S, Papadogeorgakis N. Evaluation of immunohistochemical expression of p53, p21, p27, cyclin D1, and Ki67 in oral and oropharyngeal squamous cell carcinoma. J Oral Pathol Med 2012; 41(1):40-6. doi: 10.1111/j.1600-0714.2011.01071.x [Crossref] [ Google Scholar]
- Scantlebury JB, Luo J, Thorstad WL, El-Mofty SK, Lewis JS Jr. Cyclin D1-a prognostic marker in oropharyngeal squamous cell carcinoma that is tightly associated with high-risk human papillomavirus status. Hum Pathol 2013; 44(8):1672-80. doi: 10.1016/j.humpath.2013.01.021 [Crossref] [ Google Scholar]
- Yu Z, Weinberger PM, Haffty BG, Sasaki C, Zerillo C, Joe J. Cyclin d1 is a valuable prognostic marker in oropharyngeal squamous cell carcinoma. Clin Cancer Res 2005; 11(3):1160-6. [ Google Scholar]