Avicenna J Dent Res. 14(1):1-9.
doi: 10.34172/ajdr.2022.01
Original Article
Green Tea May Reduce Dental Caries and Erosion: A Systematic Review
Seyedeh-Fatemeh Seyedjavadi-Limoodi 1  , Negar Moghaddasi 2, Farshad Khosraviani 3, *
, Negar Moghaddasi 2, Farshad Khosraviani 3, *  , Shiva Pouya 4
, Shiva Pouya 4 
Author information:
1D.D.S, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran
2D.D.S, School of Dentistry, Shiraz University of Medical Sciences, Shiraz, Iran
3D.D.S, School of Dentistry, University of California Los Angeles, California, USA
4D.D.S, School of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background: In recent years, the use of mouthwashes containing green tea (GT) extract has been reported to prevent tooth decay. In laboratory studies, GT has been shown to be effective in controlling dental plaque and erosion. The aim of this review study was to evaluate the effectiveness of GT extract in controlling tooth decay and erosion in randomized clinical trials.
Methods: By searching related keywords in the Scopus, PubMed, and WOS (Web of Science) databases, as well as searching for related studies in the Google Scholar database randomized clinical trial ( RCT) studies published in English by the end of 2019 were extracted. Then, eligible studies were carefully reviewed and the required data were extracted.
Results: A total of 12 eligible studies were included in the study. The inclusion criteria of the study were human RCT studies, English language, and GT intervention. The exclusion criteria of the study were lack of negative (placebo) or positive control group, studies examining the effect of GT derivatives, oral diseases such as periodontal disease, people undergoing orthodontic treatment, use of antibiotics at least two weeks before the study, incomplete methodology, defects in the results, and lack of access to the full text of articles. The number of subjects was 246 in the GT group, 157 in the placebo group, and 132 in the positive control group (sodium fluoride, chlorhexidine, neem extract, and probiotic). Eleven studies used GT mouthwash and one study used toothpaste containing GT extract. In studies with placebo, GT showed a significantly better effect on dental plaque, oral pH, modulation of dental erosion, and reduction of the number salivary S. mutans and lactobacilli colonies. Additionally, GT had a similar and comparable effect to positive control groups in modulating the above-mentioned indices.
Conclusion: GT extract can show anti-cariogenic and anti-erosive effects. Larger randomized clinical trials are needed to support our findings.
Keywords: Green tea, Dental caries, Dental erosion, Clinical trial
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Seyedjavadi-Limoodi S, Moghaddasi N, Khosraviani F, Pouya S. Green tea may reduce dental caries and erosion: a systematic review. Avicenna J Dent Res. 2022; 14(1):1-9. doi:10.34172/ajdr.2022.01
Background
Erosion and tooth decay are common problems in communities. Dental erosion begins with demineralization of the tooth which leads to tooth decay in advanced cases. More than 30% of adolescents and adults suffer from some degree of dental erosion, and 18% of them are in severe conditions (1). More than 40% of children and adolescents suffer from dental caries, which is more common in poor communities (2). Consumption of beverages and fruit juices, gastroesophageal reflux, acid production by oral bacterial flora, aging, and being male are among the main risk factors for tooth erosion and decay (1,3,4).
Prevention of tooth erosion and caries is the ideal way; however, many patients may refer to the dentist in advanced and symptomatic stages and the initial preventive strategy may not be effective for them. The preventive strategy includes modulation or elimination of risk factors, diagnosis and initial treatment, using remineralization agents, and patient education (5).
The products containing fluoride have shown promising results in reducing dental erosion; however, fluoride has not played a completely preventive role (6). Brushing teeth with toothpaste containing fluoride is a common way to control tooth decay, but this method is not completely able to deal with the pathology of tooth decay, especially with the removal of dental plaque. The use of mouthwashes may enhance the anti-plaque and anti-bacterial effects of the toothpaste (7). The results of clinical studies show that the use of fluoride mouthwashes has been associated with a reduction in the incidence of caries, tooth loss, and the need for dental restoration by up to 23% (8). Chlorhexidine (CHX) is another commonly used compound for preventing tooth decay and dental plaque, but it has side effects. Calculus formation, tooth and mucosa discoloration, taste disturbance, mucosal damage, and allergic reactions are among the side effects of CHX that may reduce its acceptance among people, especially in the long run (9).
In recent years, GT extract has been introduced as a protective agent against the acidic environment. Laboratory studies indicate that GT extract has a similar effect to fluoride and CHX compounds on the control of tooth decay, although more studies are needed for a definite conclusion (10-12). GT belongs to the family Camellia sinensis. It is used daily as a beverage in many countries, especially in Asia. GT leaf extract contains various chemicals. The main constituents of GT are caffeine, flavonoids, and polyphenols (13). Over the past two decades, an abundant number of studies have reported the effects of GT on health. These effects include anti-cancer effects (14), modulation of blood lipid (15) and type 2 diabetes (16), and antioxidant effects (17). The anti-bacterial effect of GT, treatment of periodontal disease (18,19) and controlling dental plaque (20) have the highest amount of evidence for the use of GT in dentistry.
The aim of this systematic review study was to evaluate the human clinical trials on the effect of GT extract on the control of dental erosion and caries.
Materials and Methods
This systematic review study examined the effect of GT extract on the factors affecting tooth decay and erosion in human clinical trials. The reporting framework of this study is consistent with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyzes) checklist (21). First, the keywords based on MeSH, and the keywords of related articles were prepared as follows: (“green tea extract” OR “Camellia sinensis extract” OR “green tea mouthwash” OR “green tea mouth rinse “) AND (“tooth demineralization” OR “remineralization” OR “dental erosion” OR “dental caries” OR “oral flora” OR “anti-bacterial” OR” bacteria” OR” anti-cariogenic “)
Then, the keywords were searched in three databases: Scopus, PubMed, and Web of Science (WOS). Based on the items in the above-mentioned databases, the search was limited to randomized clinical trial (RCT) studies published in English by the end of 2019. Then, the article headlines were extracted. Articles were searched in March 2020.
Then, the articles were electronically sent to two reviewers (S.P, F.K). The reviewers evaluated the articles using Mendeley software version 19.3 for title, abstract, and text. Additionally, a checklist was given to reviewers to classify articles according to the study criteria. Then, eligible articles were merged. Differences in the selected articles of the two reviewers were examined and interpreted by the third reviewer (S.S). To increase the sensitivity of the search, the first 50 relevant articles displayed in the Google Scholar database were reviewed and added to the study.
Inclusion criteria of the study were: Human RCT studies, English language and GT intervention.
Exclusion criteria of the study were: Lack of negative (placebo) or positive control group, studies examining the effect of GT derivatives, oral diseases such as periodontal disease, people undergoing orthodontic treatment, use antibiotics at least two weeks before the study, incomplete methodology, defects in the results reported, and lack of access to the full text of articles.
Then, the third reviewer (S.S) reviewed the final articles. Information including the type of study, number of participants, study groups, type of intervention, type and concentration of GT extract, evaluation method, study duration, and intervention results were carefully reviewed and extracted. The results of the interventions were reported to be effective (observing a significant difference between the GT group and the placebo, observing significantly superior results or not any significant difference between the GT group and the positive control group) or ineffective (not observing significant difference compared to the negative control group).
The CONSORT 2010 checklist was used to evaluate the quality of the studies (22). This checklist has 37 items. The result of the quality score of the articles was expressed as a percentage according to the items obtained from the checklist. The Cochrane Collaboration tool was used to examine the risk of bias in the studies. This checklist has five items and its bias level is classified as high (H), uncertain (U), and low (L) (23).
Results
Search Results
The article search results in the databases are shown in Figure 1. The initial search in the three databases of Scopus, PubMed, and WOS showed 4403 titles. After restricting and merging the titles, 596 titles remained which were sent to the two reviewers. Among these titles, 551 items were omitted for reasons such as unrelated studies, as well as cellular and animal studies. Among the 35 remaining titles and 10 related titles found in the Google Scholar database (n = 45), 33 articles were excluded and 12 articles were reviewed finally. The reasons for omitting the articles are shown in Figure 1. Table 1 shows a summary of the final articles.
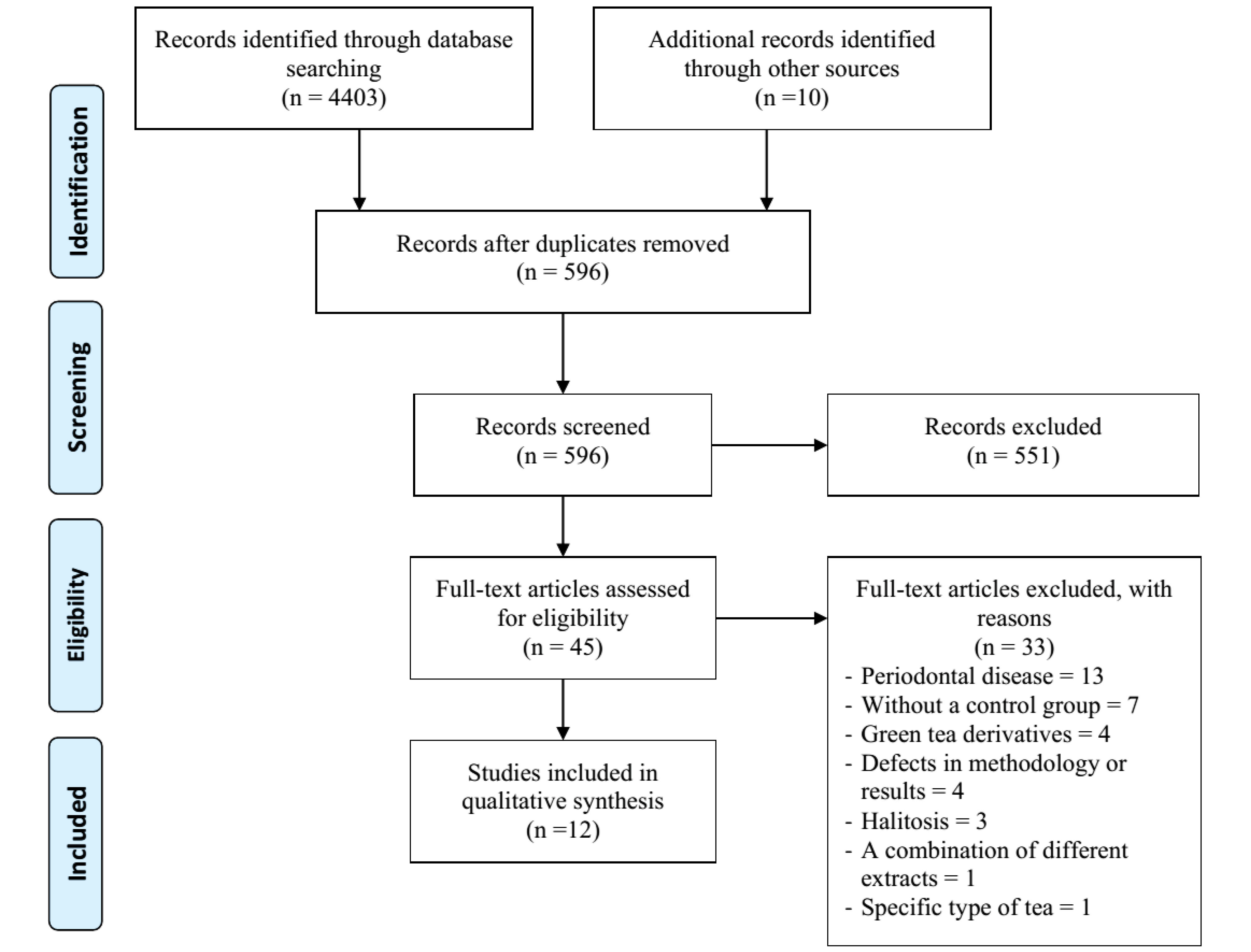
Figure 1.
PRISMA 2009 Flow Diagram of Literature Search and Study Selection.
.
PRISMA 2009 Flow Diagram of Literature Search and Study Selection.
Table 1.
A summary of the Final Articles
|
Efficacy of Green Tea
|
Type of assessment
|
Groups
|
N
|
Type of Study
|
Reference
|
| E |
S. mutans
|
GT, 1 g/100 mL
Placebo |
2×20 |
RCT |
(34) |
| E |
S. mutans
Lactobacillus
|
GT, -
CHX, 0.12%
Placebo |
3×14 |
RCT |
(33) |
| E |
S. mutans
Lactobacillus
|
GT, -
CHX, 0.12%
Probiotic
Sodium fluoride |
4×13 |
RCT |
(30) |
| E |
S. mutans
Lactobacillus
|
GT, 0.5%
CHX, 0.12%
CHX, 0.12% + sodium fluoride |
24
24
23 |
RCT |
(32) |
| E |
S. mutans
|
GT, 2 g/100 mL
CHX, 0.12%
Placebo |
3×10 |
RCT
(crossover) |
(25) |
| E |
S. mutans
Lactobacillus
|
GT, 0.5%
Sodium fluoride |
2×30 |
RCT |
(29) |
| E |
S. mutans
Lactobacillus
|
GT, 1.6 g/40 mL
placebo |
2×33 |
RCT |
(35) |
| E |
Plaque index
Gingival index |
GT, 2%
Placebo |
50
52 |
RCT |
(24) |
| E |
Plaque index
Gingival index
pH index
OHIS |
GT, 0.5%
Neem, 2%
CHX gluconate, 0.2% |
3×10 |
RCT |
(31) |
| E |
Plaque pH |
Sugar + GT, 2%
Sucrose
Milk + sugar |
3×10 |
RCT |
(26) |
| E |
Dentin erosion |
GT, 0.61%
CHX, 0.12%
Sodium fluoride
Placebo |
12 |
RCT
(crossover) |
(28) |
| E |
Dentin erosion |
GT, 0.61%
Placebo |
10 |
RCT
(crossover) |
(27) |
Abbreviations: RCT, randomized clinical trial; GT, green tea; CHX, chlorhexidine; S. mutans, Streptococcus mutans; OHIS, oral hygiene index score; E, effective.
Type of Studies and Sample Size
Among the 12 final studies, one article was a triple blind RCT (24), 8 studies were double blind RCT, one study was single blind RCT (25), and one study was open label RCT (26). One RCT study was not separable in this way due to the lack of blinding (27). Three studies had a crossover design in which, by time interval, the interventions rotated between the studies’ subjects (25,27,28). Additionally, 535 individuals were enrolled in the studies. Considering the rotation in crossover studies, the groups included: GT extract (n = 246), placebo such as water (n = 157), sodium fluoride (n = 55) (28-30), CHX (n = 69) (25,28,30-33), neem extract (n = 10) (31), combination of CHX and sodium fluoride (n = 25) (32), and probiotic (n = 13) (30). Participants in seven studies were adults and in five studies, they were under 18 years (26,29,32-34).
GT Compounds
The studies were conducted in Brazil, Egypt, India, Iran, Italy, and Saudi Arabia. The type of GT leaf used in the studies was not mentioned.
In 9 studies, aqueous GT extract was used, and in one study, the alcoholic extract was used (29). In two studies, it was not clear whether the GT extract was aqueous or alcoholic (28,30). Additionally, diluted aqueous solution of GT was used in 11 studies and GT extract in combination with toothpaste was used in one study (30). GT concentration in six studies varied between 0.5% and 2%. In four studies, the final concentration of GT was not exactly mentioned, but in their report, the amounts of dried GT leaves in boiling water were 2 g/180 mL (27), 2 g/100 mL (25), 1.6 g/40 mL (35), and 1 g/100 mL (34). In two studies, GT concentration was not calculable (30,33).
Study Groups
Positive control groups in the studies included CHX (25,28,30–33), sodium fluoride (28–30,32), neem extract (31), and probiotics (30). In the negative control group, water, sugar, and fluoride-free toothpaste were used. In 5 studies, there were only positive control groups and the GT group was compared with them (29-33). In six studies, the placebo group was compared with the GT group (24,26-28,34,35). In two studies, the placebo and positive control groups were compared with the GT group (25,28).
Efficacy Assessment of GT
Seven studies investigated the effect of interventions on S. mutans and lactobacilli, one study examined the effect of GT on plaque pH (26), two studies examined the effect of GT on dental and gingival plaque index (24,31) and two studies examined dental erosion (27,28).
Length of Studies
Intervention duration in the studies can be summarized as few hours (25,26), five days (27,28), one week (35), two weeks (29,32,33), three weeks (31), and one month (24,30,34).
Effectiveness
In all of the studies, GT showed a significantly better effect than placebo and a similar effect or superiority over positive control groups. The following is a summary of GT effectiveness results.
Anti-bacterial effect
In a study by Ferrazzano et al, participants in the GT group were asked to use GT mouthwash three times a day for a week. The control group used placebo mouthwash. Colonies of S. mutans and lactobacilli were counted in saliva. In the GT group, the levels of S. mutans and lactobacilli were significantly reduced. In this study, colony reduction was not mentioned separately and instead Odds ratio (OR) was reported. At the end of the fourth day, the ORs were recorded as 3.12 and 4.02 for S. mutans and lactobacilli, respectively, and at the end of the seventh day, these values were 3.12 and 4.02, respectively (35).
Neturi et al compared GT mouthwash with CHX and placebo. In their study, mouthwashes were given to subjects and they were asked to rinse their mouths with them (10 mL) for one minute. Before and 5 minutes after mouthwash, dental plaque was obtained for S. mutans colony count. This process was repeated one and two weeks later and each group used all mouthwashes alternately in three phases. Based on the results, the colony count in all phases of the study was significantly reduced in the CHX group (17.2-17.9%) and the GT group (16.1-17.4%) in comparison with the placebo group (-0.6 - + 0.6%). There was no significant difference between GT and CHX groups (25).
Salama and Alsughier divided 40 children into two groups: GT and placebo mouthwashes. The mouthwash was used twice daily for 4 weeks. Salivary S. mutans colonies were assessed at baseline, 2 and 4 weeks after the start of the study. The results showed a statistically significant reduction in the S. mutans count two weeks (23.2% vs. 4.5%) and four weeks (55.4% vs. 10%) after the intervention compared with placebo (34).
In the study by Ali et al, 42 children were divided into three groups: CHX, GT, and placebo mouthwashes. Subjects were asked to use 5 mL of mouthwash for two weeks (twice a day for one minute). At the end of the study, two mL of saliva was taken from the subjects and examined for S. mutans and lactobacilli. Based on the results, CHX and GT significantly reduced the bacterial colony of both types when compared to placebo. CHX and GT groups were not significantly different from each other. The rates of reductions in S. mutans and lactobacilli colonies were 50% and 46.6% in the GT group, 51.8% and 45.9% in the CHX group, and 0.3% and 1.4% in the placebo group, respectively (33).
Tehrani et al divided 60 children (6-12 years old) into groups of 0.5% GT and 0.05% sodium fluoride mouthwashes. Subjects used mouthwash twice a day for 2 weeks and at the end of the study, the colony counts of S. mutans and lactobacilli obtained from saliva samples were examined. Both interventions significantly reduced bacterial count without significant differences. In GT and sodium fluoride groups, the rates of reduction were 97.8% and 99.4% in S. mutans colonies and 72.1% and 56% in lactobacilli colonies, respectively. For each type of bacteria, no difference was observed between the two groups (29). Hegde and Kamath divided 75 children into three groups of CHX, CHX + sodium fluoride, and GT mouthwashes. Children used mouthwash daily for two weeks. First, 2 mL of saliva was collected and the number of S. mutans and lactobacilli colonies was counted before and after the study. The three groups showed a significant decrease in bacterial colony counts. By comparison, the combined and GT groups were not significantly different from each other. CHX alone had significantly better results than the other two groups (54.7% vs. 41.1% and 42%). The rates of reduction in lactobacilli count were reported to be 56.7%, 43.2%, and 37.1% in CHX, GT, and the combined groups, respectively, but no difference was observed among the groups (32).
In the study conducted by Prabakar et al, 42 subjects were divided into four groups of participants using toothpastes containing GT, sodium fluoride, CHX, and probiotic. The number of S. mutans and lactobacilli colonies in saliva and dental plaque was counted after 15 and 30 days of the interventions. Based on the results, all the interventions reduced the colony counts, but none of the groups were significantly different from each other (30).
Anti-erosive Effect
Kato et al compared the effect of GT mouthwash with water on dental erosion and dental erosion + abrasion. The participants used GT extract or water in two crossover phases. Each phase lasted five days and the interval between phases was seven days. They attached samples of bovine teeth to orthodontic appliance. During each phase, the instruments were placed in the mouth for 24 hours and were taken out of the mouth four times a day after food consumption. Then, the bovine teeth were subjected to Coca-Cola (erosion), and in some samples, the teeth were subjected to Coca-Cola and toothbrush (erosion + abrasion). After each challenge, people placed the appliance in their mouths and rinsed their mouths with mouthwashes for one minute. The amount of dentin loss was higher in the erosion + abrasion group than in the erosion group alone. GT significantly reduced erosion (39.8%) and abrasion (26.8%) when compared with placebo (27). Magalhães et al similar to Kato et al evaluated 12 volunteers in four phases (each phase lasted five days). Study groups included 250 ppm sodium fluoride, 0.12% CHX, 0.61% GT, and deionized water. Based on the results, the erosion + abrasion group had a lower enamel thickness when compared with the erosion group. All positive control groups showed decreased erosion and abrasion and this decrease was higher in the GT, the CHX, and the sodium fluoride groups, respectively, but no significant difference was observed between them. In the GT group, the rates of reductions in abrasion + erosion and erosion were 37.5% and 45%; these values were 33.3% and 40% in the CHX group, and 29.2% and 30% in the sodium fluoride group (28).
Anti-plaque Effect
Sarin et al divided 102 adults into two groups of 2% GT and placebo mouthwashes. The participants used mouthwash twice a day for 28 days. In intragroup comparison, only the GT group showed a significant decrease in plaque and gingival index. Compared with the placebo group, plaque index (48.1% vs. 12.5%) and gingival index (44.7% vs. 3.4%) showed a more significant decrease in the GT group (24). Balappanavar et al compared 0.5% GT mouthwash with neem extract and CHX mouthwashes. GT and neem were used daily for three weeks and CHX was used for two weeks. The participants were monitored before, immediately after the first wash, and every week until the end of each intervention by measuring the plaque index, gingival index, and oral health status. The plaque index had the highest to the lowest decrease in the GT, neem, and CHX groups, respectively, to the extent that the downward trend in the GT group was higher than that in the other two groups. The gingival index in all three groups showed a similar improvement. The highest reduction in the gingival index was observed in the GT group. Oral health and hygiene in the GT and neem groups had an upward trend and this index in the CHX group had an alternating trend. The best oral hygiene score was recorded for the GT group, followed by neem and CHX groups. The comparison between the groups at the end of the second week showed that the plaque index in the GT group was significantly higher compared to the other groups and there was no significant difference in terms of other indices (31).
pH Modulation Effect
In other part of their study, Balappanavar et al compared the pH of saliva after using 0.5% GT, neem, and CHX mouthwashes. The pH at the beginning of the study was about 5 in all three groups and at the end of the study, it was about 6.3 in all three groups, which showed a similar upward trend. By comparing the groups at the end of the second week, it was revealed that there was no significant difference in pH level. At the end of the third week, the GT group had a significantly higher pH than the neem group (6.44 ± 0.39 vs. 6.28 ± 0.44) (31).
Additionally, Talreja et al reported that at all times after the intervention (5, 10, 20, and 30 minutes), the pH of plaque in the GT group (6.69-6.93) was significantly higher compared to the milk + sugar group (6.45-6.50) and the sucrose group (6.01-6.16). In addition, the pH in the milk + sugar group was higher compared to the sucrose group, significantly (26).
Quality of Studies and Bias
The quality score of the studies varied between 62.5 and 90.6%. Lack of randomization and blinding process, as well as lack of access to study protocol were the main reasons for the decrease in the quality score of the articles. Among the studies, lack of randomization, blinding process, and follow-up period were the main causes of bias in the studies. Table 2 shows the quality and risk of bias distribution in the studies.
Table 2.
The Quality and Risk of Bias Assessment of the RCT Studies Based on Modified CONSORT 2010 Statement and Cochrane Risk of Bias Tool
|
Study
|
(29) |
(35) |
(31) |
(24) |
(25) |
(32) |
(26) |
(30) |
(34) |
(28) |
(33) |
(27) |
|
Quality
|
| No. positive items |
28/32 |
23/33 |
24/32 |
25/32 |
22/32 |
23/32 |
21/30 |
29/32 |
23/32 |
26/32 |
22/32 |
20/32 |
| % |
87.5 |
69.7 |
75 |
78.1 |
68.8 |
71.9 |
70 |
90.6 |
71.9 |
81.3 |
68.8 |
62.5 |
|
Bias
|
| Random sequence generation |
L |
H |
H |
L |
L |
L |
L |
L |
H |
H |
H |
H |
| Allocation concealment |
L |
H |
H |
L |
U |
L |
L |
L |
H |
H |
U |
L |
| Blinding of participants and personnel |
L |
U |
U |
L |
H |
U |
H |
L |
L |
L |
H |
H |
| Blinding of outcome assessment |
L |
U |
U |
U |
U |
L |
L |
L |
L |
L |
L |
L |
| Incomplete outcome data |
L |
L |
L |
L |
L |
L |
L |
L |
L |
L |
L |
L |
| Other (follow-up) |
H |
H |
H |
H |
H |
H |
H |
H |
H |
H |
H |
H |
H, high; U, uncertain; L, low.
Discussion
Findings of the above-mentioned 12 RCT studies showed that GT extract as a mouthwash or in combination with toothpaste can modulate factors related to tooth aging and caries including dental erosion and abrasion, salivary acidity, oral bacterial flora, and dental plaque, and they also demonstrated that GT did not have a placebo effect. GT also showed a similar anti-cryogenic effect to standard mouthwashes including CHX and sodium fluoride.
Tea is available in black, oolong (fermented), and green (without fermentation). Catechins 30%, other polyphenols 8%, minerals 10%, proteins and amino acids 9%, carbohydrates 7%, tannins 3%, and caffeine 3% are the main components of the dry leaf of GT (36). Polyphenols such as Epicatechin, Epicatechin-3-gallate, Epigallocatechin, and Epigallocatechin-3-gallate are the most important components of GT. The catechins in GT appear to have the main protective effects on tissue health (37,38). The amount of active catechins of GT decreased significantly by fermentation (37).
In seven studies, GT had a reducing effect on the colonies of S. mutans and lactobacilli in samples prepared from saliva and dental plaque (25,29,30,32-35) and in two studies, it decreased dental plaque (24,31). In cross-sectional studies, in the dental plaques accompanied by significant dental caries, S. mutans was a common dental plaque microflora, and only the lack or a lower load of this bacterium has reduced the incidence and severity of dental caries (39,40). Although dental plaque is polymicrobial, S. mutans enhance the presence of other bacteria in dental plaque (41). S. mutans break down carbohydrates by producing lactic acid and enzymes. With the fermentation and accumulation of sucrose by S. mutans, an insoluble extracellular polymer called glucan is produced, which forms the biofilm and dental plaque base and stabilizes the presence of bacteria in the plaque (42). Various species of lactobacilli are present in the oral cavity and gastrointestinal tract. The main source of Lactobacillus is dairy. In epidemiological studies, Lactobacillus has a high prevalence in people with dental caries (43). Lactobacillus has the ability to produce acid, break down sugar, and produce glucan in some species (43). It seems that GT with a concentration of 2 mg/mL prevents the formation of dental plaque completely and has an anti-bacterial effect on different species of oral microflora (44). The catechins in GT have a significant anti-bacterial effect. EGCG reduces acid production by S. mutans, inhibits the growth of S. mutans, and reduces the viability of the S. mutans colonies. In addition, it reduces the metabolic activity and virulence of S. mutans by inhibiting the activity of ATPase and lactate dehydrogenase (45). EGCG has been proven to reduce glucan production by S. mutans (46), thereby reducing the ability of S. mutants to bind to the surrounding environment (47). GT reduces the hydrolysis of starch by salivary amylase and thus reduces the availability of broken sugars to bacteria. GT inhibits bacterial amylase too (48).
In two reviewed studies, the use of GT mouthwash reduced dental erosion and abrasion up to 45% (27,28). In the laboratory, the immersion of the bovine teeth samples in the Coca-Cola, Kuat guarana, Sprite, and light Coca-Cola beverages in combination with 1.2% GT extract reduced dental erosion by 15-40% compared with the control group(49). In another laboratory study, after immersing samples of human coronary dentin in citric acid and then washing them with CHX, water, or GT, the lowest amount of erosion was observed in the GT group and CHX, respectively. Loss of dentin hardness was also lowest in the CHX and GT groups, respectively (50). The effectiveness of GT in reducing dental erosion can be affected by its anti-bacterial effect, pH modulation and also modulation in the secretion of Matrix metallopeptidases (MMPs). In cases where the tooth is exposed to endogenous or exogenous acids acutely, the role of acidity modulation and MMPs seems to be more significant. In acidic environments, the secretion of MMPs from the tissue and bacteria increases, which causes damage to collagen in the tooth tissue, a greater tooth permeability, decreased tooth strength, and increased erosion (51). GT decreases MMPs 3, 8 and 9 (52). A similar effect has been observed for CHX on the reduction of MMPs 2, 8, and 9 in the laboratory model (53). GT can also improve the eroded texture and increase the microhardness of eroded dentin with erosion (54).
Fluoride reduces demineralization and increases remineralization in a dynamic process (55) and has a protective effect on the development of erosion and tooth decay. Depending on the type of the GT and the method of extraction, there were different concentrations of fluoride in GT extract. Generally, the minimum and maximum concentrations of fluoride in the GT infusions were 0.16 mg/L and 3.29 mg/L, respectively (56). GT had a similar effect to fluoride in reducing bacterial colonization (29,30,32) and modulating dental erosion (28) in four reviewed studies. In two reviewed studies that measured the concentration of fluoride in GT, its levels were 1.4 and 0.39 ppm, compared to 221 and 250 ppm of fluoride in the fluoride mouthwash group (28,29). In these studies, the amount of GT extract fluoride was close to city water fluoride amount (about 0.7 ppm) (57) and the concentration of fluoride in 15 types of GT extract (56). Accordingly, it seems that the anti-erosive effects of GT and fluoride are due to the presence of other compounds in GT (54), which needs further studies to be confirmed. No significant side effect was found in the available literature for GT mouthwash and it seems to be safe. Among the reviewed studies, two studies evaluated the possible clinical side effects of GT extract. CHX, GT, and neem mouthwashes were reported to be acceptable to 80%, 78%, and 60% of the people, respectively (31). In addition, the color and bitterness of GT had a repulsive effect, but no side effects were recorded (31). In the study by Tehrani et al, GT extract was acceptable for participants and had no side effects (29). A pilot study, which evaluated the side effects of GT and CHX mouthwashes in two groups of 18 individuals for two weeks, showed that the mild to moderate burning sensation and dry mouth were the most common side effects in the GT group, but there was no significant difference between the groups in terms of side effects (58). The limitations of the reviewed studies included short-term interventions (one month or less), failure to follow up and evaluate the stability of the effects of interventions, failure to evaluate possible local and systemic complications of GT in most studies, and failure to evaluate GT with concentrations greater than 2%. These limitations can be reduced in future studies.
In summary, the review of 12 articles showed that the use of mouthwash and toothpaste containing GT extract has anti-bacterial, anti-plaque, and, anti-erosive effects comparable to sodium fluoride and CHX mouthwashes and can be a preventative agent against tooth erosion and decay. For a better summary, more RCT studies with larger sample size are needed. Using different concentrations of GT extract with and without combination with fluoride can show a better view of its effects on tooth decay and erosion.
Authors’ Contribution
FK and SP screened the titles and selected the qualified articles. NM was the plan supervisor. SFS was the main author. FK was the corresponding author.
Conflict of Interest Disclosures
The authors declare that there is no conflict of interest.
Ethical Statement
Not applicable.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Skalsky Jarkander M, Grindefjord M, Carlstedt K. Dental erosion, prevalence and risk factors among a group of adolescents in Stockholm County. Eur Arch Paediatr Dent 2018; 19(1):23-31. doi: 10.1007/s40368-017-0317-5 [Crossref] [ Google Scholar]
- Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol 2017; 44 Suppl 18:S94-S105. doi: 10.1111/jcpe.12677 [Crossref] [ Google Scholar]
- Tao DY, Hao G, Lu HX, Tian Y, Feng XP. Dental erosion among children aged 3-6 years and its associated indicators. J Public Health Dent 2015; 75(4):291-7. doi: 10.1111/jphd.12098 [Crossref] [ Google Scholar]
- Jaeggi T, Lussi A. Prevalence, incidence and distribution of erosion. Monogr Oral Sci 2014; 25:55-73. doi: 10.1159/000360973 [Crossref] [ Google Scholar]
- Amaechi BT, Higham SM. Dental erosion: possible approaches to prevention and control. J Dent 2005; 33(3):243-52. doi: 10.1016/j.jdent.2004.10.014 [Crossref] [ Google Scholar]
- Magalhães AC, Wiegand A, Rios D, Buzalaf MAR, Lussi A. Fluoride in dental erosion. Monogr Oral Sci 2011; 22:158-70. doi: 10.1159/000325167 [Crossref] [ Google Scholar]
- Pitts N, Duckworth RM, Marsh P, Mutti B, Parnell C, Zero D. Post-brushing rinsing for the control of dental caries: exploration of the available evidence to establish what advice we should give our patients. Br Dent J 2012; 212(7):315-20. doi: 10.1038/sj.bdj.2012.260 [Crossref] [ Google Scholar]
- Marinho VC, Chong LY, Worthington HV, Walsh T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2016; 7(7):CD002284. doi: 10.1002/14651858.CD002284.pub2 [Crossref] [ Google Scholar]
- James P, Worthington HV, Parnell C, Harding M, Lamont T, Cheung A. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev 2017; 3(3):CD008676. doi: 10.1002/14651858.CD008676.pub2 [Crossref] [ Google Scholar]
- Wang YL, Chang HH, Chiang YC, Lu YC, Lin CP. Effects of fluoride and epigallocatechin gallate on soft-drink-induced dental erosion of enamel and root dentin. J Formos Med Assoc 2018; 117(4):276-82. doi: 10.1016/j.jfma.2018.01.020 [Crossref] [ Google Scholar]
- Haripriya R, Geetha RV. Comparison of the effect of Triphala, green tea, and 2% chlorhexidine on Streptococcus mutans biofilm formation. Drug Invent Today 2019; 12(9):2034-7. [ Google Scholar]
- Kaur H, Jain S, Kaur A. Comparative evaluation of the antiplaque effectiveness of green tea catechin mouthwash with chlorhexidine gluconate. J Indian Soc Periodontol 2014; 18(2):178-82. doi: 10.4103/0972-124x.131320 [Crossref] [ Google Scholar]
- Senanayake SP. Green tea extract: chemistry, antioxidant properties and food applications – a review. J Funct Foods 2013; 5(4):1529-41. doi: 10.1016/j.jff.2013.08.011 [Crossref] [ Google Scholar]
- Seely D, Mills EJ, Wu P, Verma S, Guyatt GH. The effects of green tea consumption on incidence of breast cancer and recurrence of breast cancer: a systematic review and meta-analysis. Integr Cancer Ther 2005; 4(2):144-55. doi: 10.1177/1534735405276420 [Crossref] [ Google Scholar]
- Momose Y, Maeda-Yamamoto M, Nabetani H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int J Food Sci Nutr 2016; 67(6):606-13. doi: 10.1080/09637486.2016.1196655 [Crossref] [ Google Scholar]
- Hsu CH, Liao YL, Lin SC, Tsai TH, Huang CJ, Chou P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern Med Rev 2011; 16(2):157-63. [ Google Scholar]
- Nugala B, Namasi A, Emmadi P, Krishna PM. Role of green tea as an antioxidant in periodontal disease: the Asian paradox. J Indian Soc Periodontol 2012; 16(3):313-6. doi: 10.4103/0972-124x.100902 [Crossref] [ Google Scholar]
- Fournier-Larente J, Morin MP, Grenier D. Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. Arch Oral Biol 2016; 65:35-43. doi: 10.1016/j.archoralbio.2016.01.014 [Crossref] [ Google Scholar]
- Priya BM, Anitha V, Shanmugam M, Ashwath B, Sylva SD, Vigneshwari SK. Efficacy of chlorhexidine and green tea mouthwashes in the management of dental plaque-induced gingivitis: a comparative clinical study. Contemp Clin Dent 2015; 6(4):505-9. doi: 10.4103/0976-237x.169845 [Crossref] [ Google Scholar]
- Abdulbaqi HR, Himratul-Aznita WH, Baharuddin NA. Evaluation of Salvadora persica L and green tea anti-plaque effect: a randomized controlled crossover clinical trial. BMC Complement Altern Med 2016; 16(1):493. doi: 10.1186/s12906-016-1487-0 [Crossref] [ Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4(1):1. doi: 10.1186/2046-4053-4-1 [Crossref] [ Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340:c332. doi: 10.1136/bmj.c332 [Crossref] [ Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. doi: 10.1136/bmj.d5928 [Crossref] [ Google Scholar]
- Sarin S, Marya C, Nagpal R, Oberoi SS, Rekhi A. Preliminary clinical evidence of the antiplaque, antigingivitis efficacy of a mouthwash containing 2% green tea-a randomised clinical trial. Oral Health Prev Dent 2015; 13(3):197-203. doi: 10.3290/j.ohpd.a33447 [Crossref] [ Google Scholar]
- Neturi RS, Srinivas R, Vikram Simha B, Sandhya Sree Y, Chandra Shekar T, Siva Kumar P. Effects of green tea on Streptococcus mutans counts-a randomised control trail. J Clin Diagn Res 2014; 8(11):ZC128-30. doi: 10.7860/jcdr/2014/10963.5211 [Crossref] [ Google Scholar]
- Talreja N, Devendrappa SN, Singla SS, Agrawal N, Mali S. An in vivo comparison of plaque pH changes in children aged 8-12 years after consumption of milk and green tea with sugar. J Int Oral Health 2018; 10(1):10-5. doi: 10.4103/jioh.jioh_195_17 [Crossref] [ Google Scholar]
- Kato MT, Magalhães AC, Rios D, Hannas AR, Attin T, Buzalaf MA. Protective effect of green tea on dentin erosion and abrasion. J Appl Oral Sci 2009; 17(6):560-4. doi: 10.1590/s1678-77572009000600004 [Crossref] [ Google Scholar]
- Magalhães AC, Wiegand A, Rios D, Hannas A, Attin T, Buzalaf MA. Chlorhexidine and green tea extract reduce dentin erosion and abrasion in situ. J Dent 2009; 37(12):994-8. doi: 10.1016/j.jdent.2009.08.007 [Crossref] [ Google Scholar]
- Hajenorouzali Tehrani M, Asghari G, Hajiahmadi M. Comparing Streptococcus mutans and Lactobacillus colony count changes following green tea mouth rinse or sodium fluoride mouth rinse use in children (randomized double-blind controlled clinical trial). Dent Res J (Isfahan) 2011; 8(Suppl 1):S58-63. [ Google Scholar]
- Prabakar J, John J, Arumugham IM, Kumar RP, Sakthi DS. Comparing the effectiveness of probiotic, green tea, and chlorhexidine- and fluoride-containing dentifrices on oral microbial flora: a double-blind, randomized clinical trial. Contemp Clin Dent 2018; 9(4):560-9. doi: 10.4103/ccd.ccd_659_18 [Crossref] [ Google Scholar]
- Balappanavar AY, Sardana V, Singh M. Comparison of the effectiveness of 05% tea, 2% neem and 02% chlorhexidine mouthwashes on oral health: a randomized control trial. Indian J Dent Res 2013; 24(1):26-34. doi: 10.4103/0970-9290.114933 [Crossref] [ Google Scholar]
- Hegde RJ, Kamath S. Comparison of the Streptococcus mutans and Lactobacillus colony count changes in saliva following chlorhexidine (012%) mouth rinse, combination mouth rinse, and green tea extract (05%) mouth rinse in children. J Indian Soc Pedod Prev Dent 2017; 35(2):150-5. doi: 10.4103/jisppd.jisppd_13_17 [Crossref] [ Google Scholar]
- Moness Ali A, Ahmed WH, Abd El-Baky RM, Amer ME. Antibacterial efficacy of green tea mouth rinse in children with early childhood caries. Tanta Dent J 2019; 16(1):6-11. doi: 10.4103/tdj.tdj_41_18 [Crossref] [ Google Scholar]
- Salama MT, Alsughier ZA. Effect of green tea extract mouthwash on salivary Streptococcus mutans counts in a group of preschool children: an in vivo study. Int J Clin Pediatr Dent 2019; 12(2):133-8. doi: 10.5005/jp-journals-10005-1610 [Crossref] [ Google Scholar]
- Ferrazzano GF, Roberto L, Amato I, Cantile T, Sangianantoni G, Ingenito A. Antimicrobial properties of green tea extract against cariogenic microflora: an in vivo study. J Med Food 2011; 14(9):907-11. doi: 10.1089/jmf.2010.0196 [Crossref] [ Google Scholar]
- Harbowy ME, Balentine DA, Davies AP, Cai Y. Tea chemistry. Crit Rev Plant Sci 1997; 16(5):415-80. doi: 10.1080/07352689709701956 [Crossref] [ Google Scholar]
- Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci 2007; 81(7):519-33. doi: 10.1016/j.lfs.2007.06.011 [Crossref] [ Google Scholar]
- Chen D, Milacic V, Chen MS, Wan SB, Lam WH, Huo C. Tea polyphenols, their biological effects and potential molecular targets. Histol Histopathol 2008; 23(4):487-96. doi: 10.14670/hh-23.487 [Crossref] [ Google Scholar]
- Loesche WJ, Rowan J, Straffon LH, Loos PJ. Association of Streptococcus mutans with human dental decay. Infect Immun 1975; 11(6):1252-60. doi: 10.1128/iai.11.6.1252-1260.1975 [Crossref] [ Google Scholar]
- De Leo C, Coppola RC, Blasi G, Eftimiadi C, Salvarani M, Molina AM. Prevalence of Streptococcus mutans and dental decay in schoolchildren living in Genoa (Italy). Eur J Epidemiol 1990; 6(2):166-74. doi: 10.1007/bf00145790 [Crossref] [ Google Scholar]
- Bowen WH. Dental caries–not just holes in teeth! A perspective. Mol Oral Microbiol 2016; 31(3):228-33. doi: 10.1111/omi.12132 [Crossref] [ Google Scholar]
- Lemos JA, Palmer SR, Zeng L, Wen ZT, Kajfasz JK, Freires IA, et al. The Biology of Streptococcus mutans. Microbiol Spectr 2019; 10.1128/microbiolspec.GPP3-0051-2018.
- Caufield PW, Schön CN, Saraithong P, Li Y, Argimón S. Oral lactobacilli and dental caries: a model for niche adaptation in humans. J Dent Res 2015; 94(9 Suppl):110S-8S. doi: 10.1177/0022034515576052 [Crossref] [ Google Scholar]
- Smullen J, Finney M, Storey DM, Foster HA. Prevention of artificial dental plaque formation in vitro by plant extracts. J Appl Microbiol 2012; 113(4):964-73. doi: 10.1111/j.1365-2672.2012.05380.x [Crossref] [ Google Scholar]
- Xu X, Zhou XD, Wu CD. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob Agents Chemother 2011; 55(3):1229-36. doi: 10.1128/aac.01016-10 [Crossref] [ Google Scholar]
- Shumi W, Hossain MA, Park D-J, Park S. Inhibitory effects of green tea polyphenol epigallocatechin gallate (EGCG) on exopolysaccharide production by Streptococcus mutans under microfluidic conditions. BioChip J 2014; 8(3):179-86. doi: 10.1007/s13206-014-8304-y [Crossref] [ Google Scholar]
- Xu X, Zhou XD, Wu CD. Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Arch Oral Biol 2012; 57(6):678-83. doi: 10.1016/j.archoralbio.2011.10.021 [Crossref] [ Google Scholar]
- Zhang J, Kashket S. Inhibition of salivary amylase by black and green teas and their effects on the intraoral hydrolysis of starch. Caries Res 1998; 32(3):233-8. doi: 10.1159/000016458 [Crossref] [ Google Scholar]
- Barbosa CS, Kato MT, Buzalaf MA. Effect of supplementation of soft drinks with green tea extract on their erosive potential against dentine. Aust Dent J 2011; 56(3):317-21. doi: 10.1111/j.1834-7819.2011.01338.x [Crossref] [ Google Scholar]
- De Moraes MD, Carneiro JR, Passos VF, Santiago SL. Effect of green tea as a protective measure against dental erosion in coronary dentine. Braz Oral Res 2016;30. 10.1590/1807-3107BOR-2016.vol30.0013.
- Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res 1998; 77(8):1622-9. doi: 10.1177/00220345980770081001 [Crossref] [ Google Scholar]
- Morin MP, Grenier D. Regulation of matrix metalloproteinase secretion by green tea catechins in a three-dimensional co-culture model of macrophages and gingival fibroblasts. Arch Oral Biol 2017; 75:89-99. doi: 10.1016/j.archoralbio.2016.10.035 [Crossref] [ Google Scholar]
- Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol 1999; 6(3):437-9. doi: 10.1128/cdli.6.3.437-439.1999 [Crossref] [ Google Scholar]
- Mirkarimi M, Toomarian L. Effect of green tea extract on the treatment of dentin erosion: an in vitro study. J Dent (Tehran) 2012; 9(4):224-8. [ Google Scholar]
- Nalbantgil D, Oztoprak MO, Cakan DG, Bozkurt K, Arun T. Prevention of demineralization around orthodontic brackets using two different fluoride varnishes. Eur J Dent 2013; 7(1):41-7. doi: 10.1055/s-0039-1698994 [Crossref] [ Google Scholar]
- Maleki A, Daraei H, Mohammadi E, Zandi S, Teymouri P, Mahvi AH. Daily fluoride intake from iranian green tea: evaluation of various flavorings on fluoride release. Environ Health Insights 2016; 10:59-63. doi: 10.4137/ehi.s38511 [Crossref] [ Google Scholar]
- U.S U.S. Department of Health and Human Services Federal Panel on Community Water Fluoridation US Public Health Service recommendation for fluoride concentration in drinking water for the prevention of dental caries. Public Health Rep 2015; 130(4):318-31. doi: 10.1177/003335491513000408 [Crossref] [ Google Scholar]
- Abdulkarim R, Al-Subhi A, Bukhari R, Alkhattabi N, Mira R, Felemban O. Tolerability of a green tea-based mouth rinse: a pilot study. Saudi Dent J 2019; 31(4):457-62. doi: 10.1016/j.sdentj.2019.04.004 [Crossref] [ Google Scholar]