Avicenna J Dent Res. 15(1):5-9.
doi: 10.34172/ajdr.2023.457
Original Article
Evaluation of Antifungal Effects of Tablets containing Ginger on Dentures Contaminated with Candida (In Vitro)
Sepideh Bohlouli 1  , Safa Raeesi 2
, Safa Raeesi 2  , Hossein Samadi Kafil 3
, Hossein Samadi Kafil 3  , Ramin Negahdari 4
, Ramin Negahdari 4  , Mohammad Hassani 2
, Mohammad Hassani 2  , Zahra Aghazadeh 1, *
, Zahra Aghazadeh 1, * 
Author information:
1Department of Oral Medicine, Faculty of Dentistry, University of Tabriz Medical Sciences, Tabriz, Iran
2Under Graduate Student, Faculty of Dentistry, University of Tabriz Medical Sciences, Tabriz, Iran
3Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Prosthodontics, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background: Denture stomatitis (DS) due to Candida albicans is a chronic inflammation of mucous membranes that occurs beneath acrylic resin dentures. Various antifungal and disinfecting agents with different formulations are used to treat this condition with different side effects. Recently, the use of herbal medicines has attracted attention in the treatment of medical and dental conditions. The main goal of this study was to evaluate the antifungal efficacy of effervescent tablets containing ginger on complete dentures in patients with oral fungal infections in vitro.
Methods: In the present in vitro study, 81 acrylic resin dentures were divided into 3 groups and contaminated with Candida albicans, Candida glabrata, and Candida krusei fungal species, and each group was assigned to 3 groups, then immersed in solutions containing effervescent ginger tables, nystatin (as a positive control group), and distilled water (as a negative control group). The dentures underwent fungal culture procedures at 30-, 60-, and 180-minute intervals. Finally, the study groups were investigated for the presence or absence of fungal colonies.
Results: According to the results, the mean fungal colonies in the nystatin group were generally less than that in the ginger tablet group. The antifungal effect of nystatin began earlier than the ginger tablet, (i.e., in the presence of nystatin), and Candida counts diminished to zero after 60 minutes; however, this happened after 180 minutes in the effervescent ginger tablet solution.
Conclusions: Although the antifungal effect of nystatin was higher and faster than that of ginger-containing effervescent tablets, if necessary, it is possible to use ginger tablets for a longer time to eliminate fungal contaminants from dentures. Ginger-containing effervescent antifungal tablets require 180 minutes to exert their antifungal effect.
Keywords: Candida albicans, Candida krusei, Candida glabrata, Denture stomatitis, Nystatin
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Bohlouli S, Raeesi S, Samadi Kafil H, Negahdari R, Hassani M, Aghazadeh Z. Evaluation of antifungal effects of tablets containing ginger on dentures contaminated with candida (in vitro). Avicenna J Dent Res. 2023; 15(1):5-9. doi:10.34172/ajdr.2023.457
Introduction
Advances in medical sciences have given rise to an increase in the population of the elderly in developed and developing countries. The odds of losing teeth increase with aging. Therefore, more attention has been directed to the effect of oral health on the quality of life of the elderly. Loss of teeth affects the quality of life. It also affects food intake, speech, smile, social relations, and self-confidence (1). The effect of oral health on mental and physical health is so crucial that it has given rise to the concept of oral health-related quality of life (2). Despite the availability of treatment modalities that rely on technological advances, full dentures are still a treatment modality to replace lost teeth (3). The denture surface is prone to the formation of poly-microbial biofilms, which mature over time and become visible in the form of plaques, resulting in a local inflammatory process such as erythema and hyperplasia. Trauma is one of the most common problems of this treatment, which, in turn, predisposes to inflammation and the formation of recurrent infection (4).
Denture stomatitis (DS) is the chronic inflammation of mucous membranes beneath the denture base. The condition is found in 11%-76% of removable denture measures. Candida albicans is the most important microorganism involved in the pathogenicity of DS (5,6). Various treatment modalities have been suggested to resolve fungal infections in the DS treatment, including the application of local and systemic antifungal agents, along with the irrigation of dentures with saltwater, sodium hypochlorite solution, chlorhexidine mouthwash, and other antimicrobial mouthwashes (7).
Several systemic antifungal agents have been introduced with various formulations, including nystatin, fluconazole, and itraconazole. These medications lead to persistent and resistant infections with long-term use. Further, they have an unfavorable taste, and some of them have side effects such as diarrhea, nausea, and abdominal pain, and if such symptoms continue, a physician should be consulted in this regard (8,9). The use of mouthwashes is common, each with complications. Chlorhexidine gluconate mouthwash causes a change in the taste and discoloration of restorations and prostheses in the elderly (10). Sodium hypochlorite solution is another disinfecting agent, prescribed to patients wearing removable dentures. This agent causes oral complications when it contacts the oral mucosa, necessitating supportive treatment in addition to its effect on the denture’s surface characteristics (11). Therefore, researchers have attempted to produce or find effective antifungal agents with no side effects with a potential of general popularity. In this context, herbal medicines have attracted researchers’ attention (12,13).
Ginger is a well-known plant which is called with the scientific name of Zingiber officinale (14). There is evidence on evaluating the inhibition influence of ginger on pathogenic microorganisms. Studies have shown the positive antimicrobial results of the effect of this plant on Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli (8). In addition, CMUAc130 in the structure of ginger has been reported to be responsible for its significant inhibitory effect on some fungi, including C. albicans and C. fusarium (8,15).
Recent studies have examined the effect of ginger extract on oral infections. Ginger extract-containing mouthwash exhibited an inhibitory effect in vitro and clinical studies on fungal infections (9,14,16).
The current study investigated the antifungal effect of a new form of ginger extract on inhibiting the fungal contamination of dentures. Considering the easy use of effervescent tablets in daily routines, especially by the elderly, the present study evaluated the effect of ginger extract-containing mouthwashes on the inhibition of C. albicans growth and proliferation on full dentures in vitro.
Materials and Methods
Preparation of Effervescent Tablets Containing the Ginger Extract
Tablet components containing sodium bicarbonate, sodium carbonate, acid citric, potassium mono-persulfate, sodium benzoate, disodium edentate, sodium perborate and sodium lauryl sulfate were mixed using direct condensation. Then, the ginger extract at a minimum inhibitory concentration (MIC) = 10 mg/mL was added according to previous research (16). The ginger single concentration in all effervescent tablets was 25% (17). The components were mixed using a rotary machine to achieve a homogenous powder, which was turned into tablets using the direct condensation technique. Mannitol was used as a binding agent.
Preparation of Acrylic Resin Dentures and Bacterial Culture on Their Surface
Twenty-seven acrylic resin dentures were fabricated by taking impressions from an edentulous maxillary model using Acrosun acrylic resin powder (Beta Dent Company, serial No. 670050063). The dentures were duplicated for complete adaptation to each other. A standard laboratory procedure was employed for the fabrication of dentures. C. albicans and Candida glabrata (C. glabrata), as common species responsible for DS, and Candida krusei, as a treatment-resistant species, which were procured from the Iranian Pasteur Institute with the identification codes 89-1000, 89-1456, and 50271, respectively. The samples were cultured and proliferated on Sabouraud dextrose agar containing 4% glucose, 1% peptone, and 1.5% agar. The colonies of each fungal species were inoculated into three beakers containing 50 mL of sterile distilled water at a concentration of 108 CFU/mL. This concentration is equal to the mean colony counts cultured from the dentures of patients with DS (18). The acrylic resin dentures in each group (n = 27) were immersed in beakers for 24 hours so that the fungal species would contaminate their surface. Then, the dentures were retrieved and divided into three groups to evaluate the effect of ginger extract-containing effervescent tablets. Nystatin and distilled water were considered positive and negative controls, respectively.
Use of Antifungal Agents on Contaminated Dentures
In the nystatin group, 40 drops of nystatin (Mycostatin 100 000 IU/mL) was applied to the denture surface. In the ginger group, a ginger extract-containing effervescent tablet was dissolved in a glass of water, and the tablet was applied on the denture surface. Further, a glass of distilled water was employed in the distilled water (Sabalan, Iran) group. The contaminated dentures were exposed to materials for 30, 60, and 180 minutes. At the above-mentioned intervals, a sterile swab was used to take samples from the denture surfaces, which were transferred to culture media and underwent incubation. After 48 hours, the plates were analyzed for the growth of fungal colonies. All the laboratory procedures were conducted by one operator, and the procedures were repeated three times to decrease individual errors.
Statistical Analysis
The distribution of the data was normal, and one-way analysis of variance (ANOVA) test was applied to compare the antifungal properties between the groups. Finally, the obtained data were analyzed using SPSS-17, and statistical significance was considered P < 0.05.
Results
The results of the inhibition of fungal colony growth at 30-, 60-, and 180-minute intervals compared to the baseline (before placing in an environment with antifungal agents) are as follows:
30-Minute Interval
At the 30-minute interval, nystatin and ginger did not exhibit any significant effects on decreasing the growth and proliferation of C. albicans and C. glabrata; however, C. krusei became sensitive to nystatin at this interval, and its colonies decreased significantly in the nystatin group. Therefore, the antifungal effect of nystatin on C. krusei began at 30-minute interval.
60-Minute Interval
At the 60-minute interval, the growth and proliferation of all the fungal species significantly decreased under the effect of nystatin and ginger. Therefore, a period of 60-minutes was required for the effect of nystatin on C. albicans and C. glabrata. At this time interval, the growth and proliferation of C. glabrata diminished to zero in the presence of nystatin, and nystatin completely inhibited this fungal species in 60 minutes.
The antifungal effect of ginger-containing effervescent tablets began at 60 minutes. Thus, the minimum time necessary for these tablets to begin their effect was 60 minutes.
180-Minute Interval
At the 180-minute interval, the growth and proliferation of C. albicans and C. glabrata reduced to zero in the nystatin group, indicating complete inhibition. In the effervescent tablet group, the colony counts of C. albicans and C. krusei decreased significantly but did not diminish to zero.
It was concluded that:
-
The antifungal effect of nystatin was higher and more effective than that of ginger, and the effect began earlier than that of ginger.
-
At least one hour was necessary for the initiation of the antifungal effects of nystatin and ginger-containing effervescent tablets.
-
Neither nystatin nor ginger exhibited any antifungal effects on C. albicans at the 30-minute interval, and nystatin only decreased the growth and inhibition of C. krusei (Table 1).
Table 1.
Comparison the Effects of Nystatin and Ginger on Different Fungal Species at 30-, 60-, and 180-Minute Intervals
| The effect of ginger on different fungal species |
After 30 minutes |
P = 0.10 |
| After 60 minutes |
P = 0.09 |
| After 180 minutes |
P = 0.07 |
| The effect of nystatin on different fungal species |
After 30 minutes |
P = 0.12 |
| After 60 minutes |
P = 0.14 |
| After 180 minutes |
P = 0.11 |
These results are demonstrated in related tables and charts. Based on the results (Table 1), neither nystatin nor ginger represented any antifungal effects on C. albicans after 30 minutes, and at least a 180-minute interval was necessary for ginger to begin its effects. Further, overall, the mean colony counts of the fungal species were lower than those of nystatin.
According to Figures 1, 2, and 3, overall, nystatin was more effective in decreasing the growth of C. albicans colonies. In addition, the antifungal effect of ginger began after 30-60 minutes.
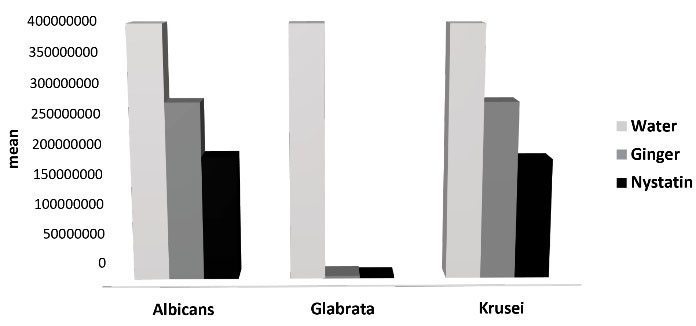
Figure 1.
Mean of Variables in 30 Minutes.
.
Mean of Variables in 30 Minutes.
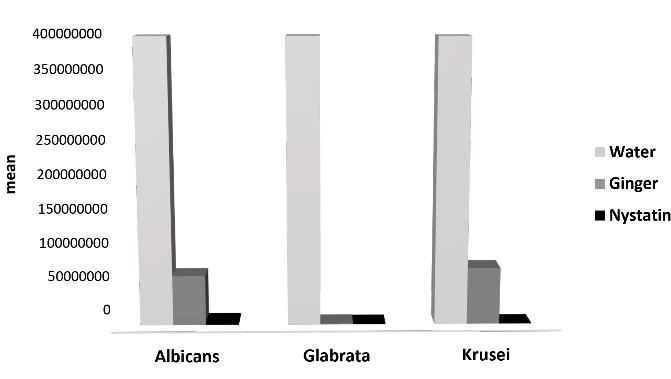
Figure 2.
Mean of Variables in 60 Minutes.
.
Mean of Variables in 60 Minutes.
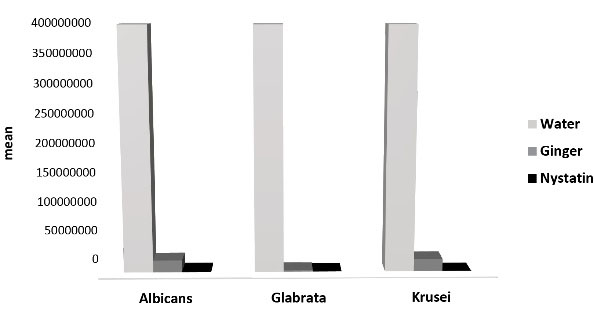
Figure 3.
Mean of Variables in 180 Minutes.
.
Mean of Variables in 180 Minutes.
Discussion
In the present study, ginger-containing effervescent tablets were produced, and their dose and the required time for their effect were evaluated in vitro. According to the results, the immersion of contaminated dentures for 3 hours in 50 mL of water with one ginger-containing tablet was enough to eliminate fungal contamination.
The inhibitory effect of ginger on the growth and proliferation of different fungal species has been investigated in different studies. However, these studies have rarely clinical views and have not been conducted in health and therapeutic product frameworks. In the current study, the ginger extract was prepared at an effective concentration achieved in previous studies in the form of ginger-containing effervescent tablets. The time necessary for the effect of these tablets on the elimination of fungal contamination from dentures was 3 hours.
The antifungal effects of the protein found on the ginger rhizome on some fungal species have been shown in vitro, including, Fusarium oxysporum(19), Fusarium moniliforme, Aspergillus flavus, and Aspergillus fumigatus (6). The inhibitory effect of ginger on the growth and proliferation of phytopathogenic fungi has been attributed to the CMUAc130 agent (15). Moreover, the ginger extract was effective in the growth inhibition of fungal species, which are resistant to the treatment with amphotericin B and ketoconazole (20). In a similar study, the ginger extract demonstrated an inhibitory influence on the growth and proliferation of C. albicans resistant to fluconazole (21).
Likewise, Aghazadeh et al evaluated the antifungal, antimicrobial, anti-biofilm, and cytotoxicity properties of the ginger extract on Candida and some other bacterial species and showed that the ginger extract had antifungal and anti-biofilm effects at 0.625-5 mg/mL concentrations. Additionally, these concentrations are safe for gingival fibroblasts and are noncytotoxic (16). Taghavi et al also investigated the antifungal effects of the ginger extract on C. glabrata and C. krusei and found that the extract in the form of a mouthwash exhibited significantly higher antifungal activity compared to the control groups consisting of fluconazole and nystatin (21). Similarly, Eslami et al examined the effect of ginger extract in the form of mouthwash on patients with DS in a clinical trial and concluded that its effect was similar to that of nystatin drops (22). Moreover, Abbasi et al evaluated the potential of the antifungal effect of ginger extract and essence solution on Candida and reported that the essence solution was more effective and exerted similar inhibitory effects at lower MICs compared to the extract (23).
Based on the available data, the unpleasant taste, side effects, and microbial resistance are the most common factors for patients’ lack of interest in using nystatin and other available local and systemic synthetic medications for DS and other fungal infections in the oral cavity. This is especially important in elderly patients who have more susceptible physical conditions. The use of mouthwashes and ointments with unpleasant taste is difficult and occasionally life-threatening due to physical limitations and neuromuscular weaknesses. On the other hand, complete edentulism is a problem that has affected a high percentage of the elderly, especially in developing countries.
Fungal infections are among the most common problems in denture wearers that occur after a while. Immune system conditions, mineral and vitamin deficiency, iron and vitamin B12 deficiency, serrated structure, improper polish of dentures, and ill-fitting dentures are some of the most common conditions that predispose the elderly to these infections (24).
The use of herbal compounds to inhibit microbial and fungal infections has attracted attention all over the world. Studies have shown an increase in patients’ interest in using herbal medicines in the past three decades. It appears that 80% of the world’s population trust herbal medicines. However, there have always been concerns about their inadvertent use and medicinal interferences (25). In the present study, effervescent tablets containing ginger were evaluated at doses determined in previous studies, and their effective time was compared with that of nystatin. Based on the results, when these tablets were dissolved in 50 mL of water for 3 hours, they could exert their antifungal effects and destroy fungal colonies on the denture surface.
Acknowledgements
The process of preparing ginger tablets was kindly performed in the Laboratory of Health and Cosmetics, Rojin Company. The authors would like to appreciate this company for its kind help and cooperation.
Author’s Contribution
Conceptualization: Zahra Aghazadeh, Sepideh Bohlouli.
Data curation: Safa Raeesi, Mohammad Hassani.
Formal Analysis: Hossein Samadi Kafil, Ramin Negahdari.
Funding acquisition: Zahra Aghazadeh, Hossein Samadi Kafil.
Investigation: Hossein Samadi Kafil, Safa Raeesi, Mohammad Hassani.
Methodology: Zahra Aghazadeh, Hossein Samadi Kafil.
Project administration: Zahra Aghazadeh, Sepideh Bohlouli.
Resources: Zahra Aghazadeh, Sepideh Bohlouli, Hossein Samadi Kafil.
Software: Ramin Negahdari.
Supervision: Zahra Aghazadeh, Sepideh Bohlouli.
Validation: Safa Raeesi, Mohammad Hassani.
Visualization: Ramin Negahdari.
Writing – original draft: Safa Raeesi, Zahra Aghazadeh, Hossein Samadi Kafil.
Writing – review & editing: Zahra Aghazadeh, Safa Raeesi.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The protocol of this in vitro study was approved by the Ethics Committee of Tabriz University of Medical Sciences under the code IR.TZMED.REC.1396.958. This study complied with the ethical standard recommended by Tabriz University of Medical Sciences.
Funding
This work was supported by a private and personal grant, and no governmental or industrial grant was used for this study.
References
- Nitschke I, Müller F. The impact of oral health on the quality of life in the elderly. Oral Health Prev Dent 2004; 2 Suppl 1:271-5. [ Google Scholar]
- Wong MC, Lo EC, McMillan AS. Validation of a Chinese version of the Oral Health Impact Profile (OHIP). Community Dent Oral Epidemiol 2002; 30(6):423-30. doi: 10.1034/j.1600-0528.2002.00013.x [Crossref] [ Google Scholar]
- Bakhshi M, Taheri JB, Shabestari SB, Tanik A, Pahlevan R. Comparison of therapeutic effect of aqueous extract of garlic and nystatin mouthwash in denture stomatitis. Gerodontology 2012; 29(2):e680-4. doi: 10.1111/j.1741-2358.2011.00544.x [Crossref] [ Google Scholar]
- Hannah VE, O’Donnell L, Robertson D, Ramage G. Denture stomatitis: causes, cures and prevention. Prim Dent J 2017; 6(4):46-51. doi: 10.1308/205016817822230175 [Crossref] [ Google Scholar]
- Shafer WG, Hine MK, Levy BM. Text Book of Oral Pathology. 4th ed. Philadelphia: WB Saunders Company; 1983.
- Zarb GA, Bolender CL, Eckert SE, Jacob R, Fenton A, Mericske-Stern R. Prosthodontic treatment for edentulous patients. 12th ed. Mosby. 2005. p. 195-7.
- Greenberg MS, Glick M, Ship JA. Burket’s Oral Medicine: Diagnosis and Treatment. 11th ed. Hamilton: BC Decker; 2008. p. 178-82.
- Momeni l, Zamanzad B. The antibacterial properties of Allium cepa (onion) and Zingiber officinale (ginger) extracts on Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Candida albicans isolated from vaginal specimens. J Shahrekord Univ Med Sci 2010;11(4):81-7. [Persian].
- Lyu X, Zhao C, Yan ZM, Hua H. Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des Devel Ther 2016; 10:1161-71. doi: 10.2147/dddt.s100795 [Crossref] [ Google Scholar]
- McCoy LC, Wehler CJ, Rich SE, Garcia RI, Miller DR, Jones JA. Adverse events associated with chlorhexidine use: results from the Department of Veterans Affairs Dental Diabetes Study. J Am Dent Assoc 2008; 139(2):178-83. doi: 10.14219/jada.archive.2008.0134 [Crossref] [ Google Scholar]
- Slaughter RJ, Watts M, Vale JA, Grieve JR, Schep LJ. The clinical toxicology of sodium hypochlorite. Clin Toxicol (Phila) 2019; 57(5):303-11. doi: 10.1080/15563650.2018.1543889 [Crossref] [ Google Scholar]
- Scorzoni L, de Paula ESAC, Marcos CM, Assato PA, de Melo WC, de Oliveira HC. Antifungal therapy: new advances in the understanding and treatment of mycosis. Front Microbiol 2017; 8:36. doi: 10.3389/fmicb.2017.00036 [Crossref] [ Google Scholar]
- McKeny PT, Nessel TA, Zito PM. Antifungal antibiotics. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022.
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol 2008; 46(2):409-20. doi: 10.1016/j.fct.2007.09.085 [Crossref] [ Google Scholar]
- Lewis RE. Current concepts in antifungal pharmacology. Mayo Clin Proc 2011; 86(8):805-17. doi: 10.4065/mcp.2011.0247 [Crossref] [ Google Scholar]
- Aghazadeh M, Zahedi Bialvaei A, Aghazadeh M, Kabiri F, Saliani N, Yousefi M. Survey of the antibiofilm and antimicrobial effects of Zingiber officinale (in vitro study). Jundishapur J Microbiol 2016; 9(2):e30167. doi: 10.5812/jjm.30167 [Crossref] [ Google Scholar]
- Saptiwi B, Mardiati E. Antiseptics and Deodorizing Mouth Rinse with Red Ginger Juice (Zingiber officinale var. Rubrum) at Various Concentrations. In: 5th International Conference on Health Sciences (ICHS 2018). Atlantis Press; 2019.
- Shimizu K, Shimizu F, Kamiyama K. Microbiological studies on denture-induced stomatitis in children. Pediatr Dent 1987; 9(4):304-7. [ Google Scholar]
- Chen JC, Huang LJ, Wu SL, Kuo SC, Ho TY, Hsiang CY. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J Agric Food Chem 2007; 55(21):8390-7. doi: 10.1021/jf071460f [Crossref] [ Google Scholar]
- Al-Saadi MH. Effectiveness of chemical and microwave disinfection on denture biofilm fungi and the influence of disinfection on denture base adaptation. J Indian Prosthodont Soc 2014; 14(Suppl 1):24-30. doi: 10.1007/s13191-014-0354-2 [Crossref] [ Google Scholar]
- Mohammadi R, Moattar F. Antifungal activity of Zingiber officinale Rosc. essential oil against fluconazole resistant vaginal isolates of Candida albicans. J Med Plants 2007;6(24):22-7. [Persian].
- Taghavi A, Pourzare S. Comparing the Effectiveness of Ginger with Fluconazole and Nystatin on Glabrta and Cruise Candidates [thesis]. Tabriz University of Medical Sciences; 2012.
- Eslami H, Pakroo S. Comparing the Effectiveness of Ginger Mouthwash and Mouth Rinse, Denture Treatment Astyvmatys Nystatin [thesis]. Tabriz University of Medical Sciences; 2012.
- Bhattacharya PT, Misra SR. Effects of iron deficiency on the oropharyngeal region: signs, symptoms, and biological changes. In: Handbook of Famine, Starvation, and Nutrient Deprivation. Springer; 2017. p. 1-18.
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2014; 4:177. doi: 10.3389/fphar.2013.00177 [Crossref] [ Google Scholar]