Avicenna J Dent Res. 13(3):92-96.
doi: 10.34172/ajdr.2021.18
Original Article
Evaluation of Soft and Hard Tissue Condition After Dental Implant Surgery in Diabetic and Non-diabetic Patients (6 Months Follow up)
Arezoo Khabazian 1  , Ali forouzanfar 2, Hossein Parsaee 3, Samaneh Soltani 4, *
, Ali forouzanfar 2, Hossein Parsaee 3, Samaneh Soltani 4, * 
Author information:
1Assistant Professor Deptartment of Periodontics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2Assistant Professor Deptartment of Periodontics, School of Dentistry, Mashad University of Medical Sciences, Mashhad, Iran.
3Private Dentist.
4Assistant Professor Deptartment of Periodontics, School of Dentistry, Qom University of Medical Sciences and Health Services, Qom, Iran.
*
Correspondence to Samaneh Soltani, Tel: +98(031)36699110, Fax: +9803536250334, Email:
Sas_851050@yahoo.com
Abstract
Background: Hyperglycemia in diabetic patients can affect the success of many dental treatments. Thus, many dental procedures are contraindicated in patients with uncontrolled diabetes mellitus (DM) due to the consequent delay in wound healing. This study aimed to assess the effect of a long-term control of blood sugar on tissue healing after implant placement.
Methods: This cohort study evaluated 20 patients aged 50-60, referring to the School of Dentistry, Mashhad University of Medical Sciences for implant placement. All patients underwent blood sugar test and were divided into two groups of diabetic and non-diabetic patients regarding their HbA1c level. Bone loss, bleeding on probing (BOP), and pocket probing depth (PPD) of patients were measured 1 and 6 months after the implant placement. Data were analyzed using independent t test and chi-square test.
Results: Blood sugar control had no significant effect on bone loss, BOP and PPD one and six month(s) after implant placement (P>0.05). Although PPD significantly increased in both groups over time (P=0.016 in the healthy group and P=0.007 in the diabetic group), the difference between the two groups was not significant (P>0.05).
Conclusion: According to the results from this study, blood sugar control examined in the age range of our study had no significant effect on tissue healing one and six month(s) after the implant placement. However, further studies are required to explore this subject more thoroughly.
Keywords: Diabetes mellitus, Bleeding on probing, Probing depth, Bone loss
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Khabazian A, Forounzanfar A, Parsaee H, Soltani S. Evaluation of Soft and Hard Tissue Condition After Dental Implant Surgery in Diabetic and Non-diabetic Patients (6 Months Follow up). Avicenna J Dent Res. 2021;13(3):92-96. doi: 10.34172/ajdr.2021.18.
Background
Highlights
Diabetes mellitus (DM) is a metabolic disease characterized by hyperglycemia, which may be due to decreased secretion of insulin from the pancreas, development of insulin resistance, or both, along with increased production of glucose by the liver (1). DM, known as the silent epidemic of the current era, is one of the most important public health dilemmas worldwide. Due to its similarity to the plague epidemic in the 14th century, DM is also referred to as the black death of the 21st century (2). DM is classified into two types, namely types I and II. Type II DM is more common, accounting for about 90% to 95% of diabetic patients worldwide. This type is more common in adults (3). Insulin resistance is the main mechanism of development in type II DM (4). Chronic complications of DM include microvascular disorders (nephropathy, retinopathy and neuropathy) and macrovascular or cardiovascular complications (hypertension, coronary artery disease, peripheral vein disease, and cerebral vein disease) (5). Evidence shows that blood sugar control in DM patients decreases the chronic side effects (6). Also, diabetic patients with poor blood sugar control are more susceptible to oral complications of DM such as periodontal disease (7). On the other hand, diabetic patients experience poor wound healing and higher frequency of postoperative infections. Thus, non-surgical and more conservative treatment approaches should be preferably considered for diabetic patients (8). A mutual interaction exists between DM and periodontal disease. Treatment of periodontal disease has an impact on DM control and its complications (9). On the other hand, DM should be taken into account in dental treatment planning such as placement of dental implants (10). Dental implant treatment is a suitable modality for replacement of the lost teeth. It is currently the treatment of choice for many edentulous patients due to the decreased need for tooth preparation in partial edentulism, increased retention and stability in complete edentulism, improved esthetics and mastication power, and increasing the quality of life. The popularity of dental implants is increasing due to the high success rate of implant-supported restorations (83% in the maxilla and 94%in the mandible) (11). Mellado-Valero et al reviewed several studies and concluded that hyperglycemia had a negative effect on formation and regeneration of bone and decreased the bone-implant contact area. They also concluded that maintaining the blood glucose level within the normal range would enhance osseointegration and increase the success of implant treatment (12). Gomez-Moreno et al (2014) evaluated peri-implantitis in type II diabetic patients, and assessed the changes in peri-implant tissue in patients with variable levels of hyperglycemia. They measured the pocket probing depth (PDD), bleeding on probing (BOP), and marginal bone loss to generally assess the peri-implant tissue health. They found that implant treatment in diabetic patients could yield predictable results if their blood sugar was controlled through assessing the level of HbA1c (13).
Eskow et al evaluated the survival rate of dental implants during the clinical follow-ups of patients with poorly controlled type II DM for a 2-year period. The results showed that complications were directly correlated with the number of implants, and no association was detected between the level of HbA1c and occurrence of complications such as mucositis. They reported that poorly controlled type II diabetic patients had implant treatments with high success rate and limited complications (14). In a review study by Monje et al in 2017, it was reported that hyperglycemia was associated with high risk of peri-implantitis in non-smokers but such a correlation with peri-implant mucositis was not found (15). Ormianer et al (2018) evaluated the success of dental implants and occurrence of peri-implantitis in diabetic patients. They found no significant difference in survival rate between diabetic and non-diabetic patients (16).
This study, therefore, aimed to assess the effect of a long-term control of blood glucose based on the level of HbA1c on tissue healing after a dental implant placement, in order to take a step forward in management of oral and dental problems in diabetic patients and to increase their quality of life.
Materials and Methods
This prospective cohort study evaluated 20 patients between 50-60 years of age, referring to the Dentistry School of Mashhad University of Medical Sciences for a dental implant placement in the maxilla between December 2018 and June 2019. The reason behind selecting the given age range for this study were the risk of complications of type II diabetes in older patients, as well as the higher occurrence of edentulism and higher need for dental implant placement in older ages. Sampling was targeted, and the sample size was calculated to be 6 subjects for each group according to a study by Aguilar-Salvatierra et al (17), assuming the mean and standard deviation of marginal bone loss in subjects with HbA1c ≤6 and >6 to be 0.51±0.19 and 1.02±0.31 mm, respectively; and using the formula for the comparing the means, type one error of 5%, and type two error of 20%. Considering the possible dropouts and to ensure the accuracy, 10 samples were included in each group. The subjects in the two groups were matched in terms of gender. All patients underwent surgical implant placement by one surgeon at the specialty clinic of School of Dentistry, Mashhad University of Medical Sciences. Prior to the surgical procedure, proper oral hygiene was also instructed to patients. The same implant type (Straumann, Switzerland) was used for all patients. After implant placement, healing abutment was applied. All patients signed informed consent forms prior to the study enrollment and the surgery. The inclusion criteria for the control group included no cigarette smoking, absence of systemic diseases, not requiring bone augmentation surgery, and not taking medications affecting the bone metabolism. The inclusion criteria in the test group were all above-mentioned criteria plus definite diagnosis of DM and history of DM for a minimum of 2 years with HbA1c<9. Patients were monitored by an internal medicine specialist and oral pills were used to treat diabetes.
The exclusion criterion was not showing up for the follow-up sessions scheduled in one and six month(s) after implant placement. The patients were divided into two groups of healthy and hyperglycemic based on their blood glucose level and according to a study by Aguilar-Salvatierra et al (17). Subjects without history of diabetes and with HbA1c ≤6 were assigned to the healthy group (n=10), while diabetics patient with HbA1c > 6 and less than 9 were assigned to the diabetic group (n=10). The clinical periodontal parameters including BOP (quantitatively), PPD (quantitatively), and presence/absence of bone loss (qualitatively) were determined one and six month(s) after implant placement. Following Aguilar-Salvatierra et al (17) and for assessing bone loss in one and six month(s), periapical radiographs were obtained using the parallel technique. In order to measure the HbA1c, the subjects underwent blood test prior to implant placement, and had another test one and six month(s) after implant placement for gaining an assurance that no change would occur in hbA1c level. As for assessing periodontal disease, PPD and presence/absence of bone loss were evaluated according to Aguilar-Salvatierra et al (17).
Six points around each implant (3 in the vestibular side and 3 in the lingual/palatal side) were probed using a plastic periodontal probe, and the number of bleeding points was counted (17).
For measuring PPD, six points around each implant (three in the buccal and in the lingual/palatal) were probed by a plastic periodontal probe (17).
Bone loss: Two periapical radiographs were taken immediately after implant placement and after 6 months, and then they were compared (17). Presence or absence of marginal bone loss was investigated in both groups.
Data were analyzed using SPSS software version 22 using independent t test and chi-square test (α=0.05).
Results
In this study, PPD, percentage of BOP, and bone loss were measured in 20 patients including 8 females (40%) and 12 males (60%) with a mean age of 55±3.61 years (range 50-60 years) in two non-diabetic and diabetic groups one and six month(s) after implant placement. The groups were not significantly different regarding the demographic variables of age (P = 0.146) and gender (P = 0.65).
As shown in Table 1, the mean PPD for 1 month and 6 months after implant placement as for two groups were not significantly different (P > 0.05). PPD for 6 months was significantly higher than that for 1 month in both non-diabetic (P = 0.016) and diabetic (P = 0.007) groups.
Table 1.
Comparison of the mean PPD (in millimeters) in the two groups at different time points
|
Variable
|
Number
|
Non-diabetic
|
Diabetic
|
P
Value
a
|
|
Mean ± SD
|
Mean ± SD
|
| PPD at 1 month |
10 |
1.69±0.41 |
1.82±0.59 |
0.589 |
| PPD at 6 months |
10 |
1.85± 0.44 |
2.19±0.80 |
0.253 |
|
P valueb |
|
0.016 |
0.007 |
|
a In dependent t test; b Paired t test.
The mean BOP for 1 month after implant placement was almost the same as for both groups with no significant difference (P = 0.720). In non-diabetic group, BOP for 6 months after implant placement was similar with the BOP for 1 month (P = 1.0). In diabetic group, however, BOP for 6 months decreased compared to the BOP for 1 month, but the difference was not significant (P = 0.739).
In 1 month, no bone loss was observed in any of the groups. Thus, no statistical analysis was performed for bone loss in 1 month. However, bone loss was detected in both groups in 6 months (3 cases in non-diabetic group, and 2 cases in diabetic group), which was greater in non-diabetic group; but this difference was not significant (P > 0.05, Figure 1).
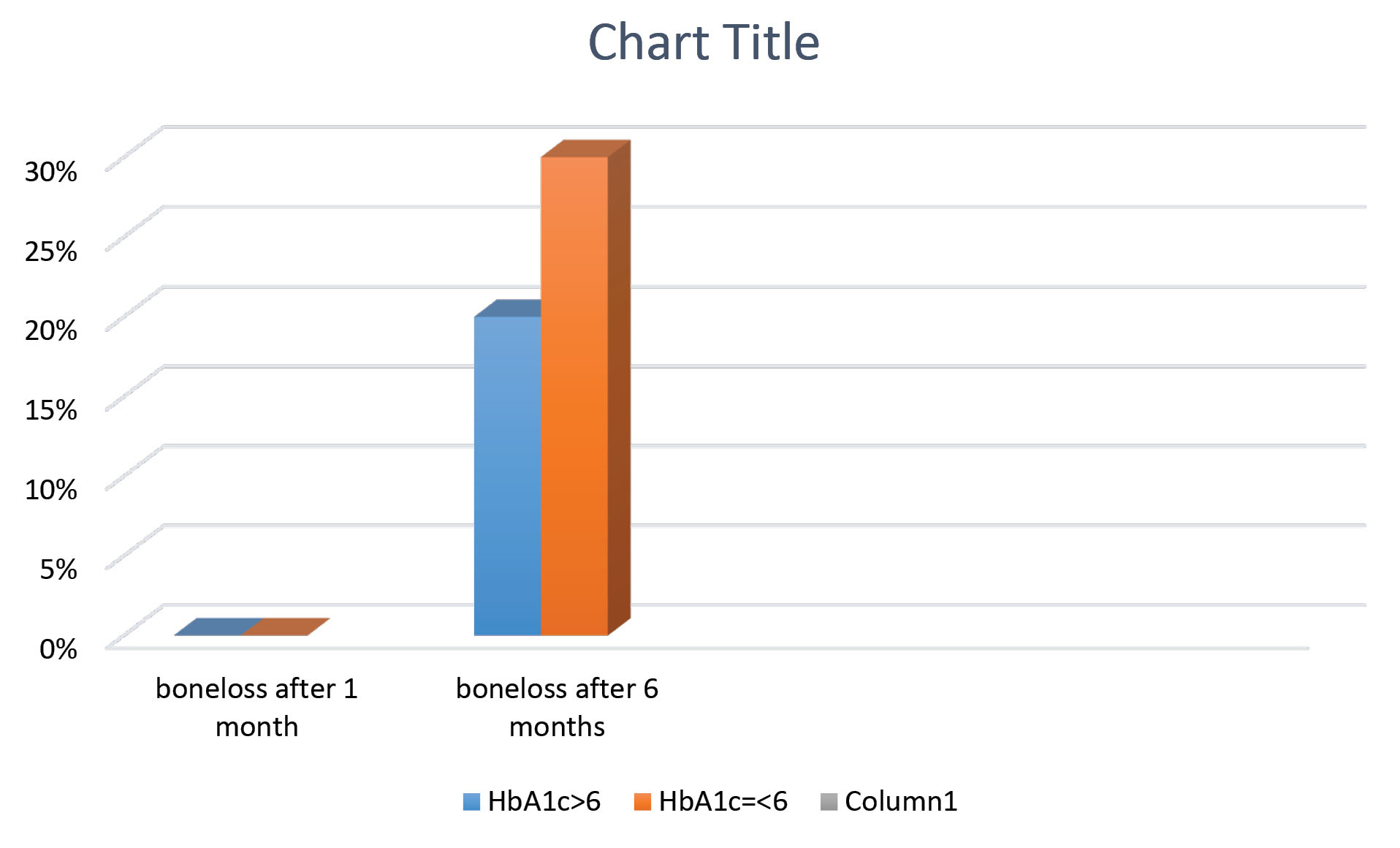
Figure 1.
Mean bone loss 1month and 6 month after implant surgery in two groups
.
Mean bone loss 1month and 6 month after implant surgery in two groups
Discussion
Regarding the concerning increase in the prevalence of obesity, the prevalence of type II diabetes is also on the rise among adults and even children. Type II diabetic patients are not necessarily obese; however, weight gain often occurs in many patients prior to development of type II DM (18). Hyperglycemia leads to infection, poor wound healing, and a compromised immune system (19). This study aimed to assess the bone loss, BOP and PPD one and six month(s) after the implant placement in diabetic patients and healthy control group. The results showed that marginal bone loss, BOP, or PPD one and six month(s) after the implant placement was not significantly different between diabetic and non-diabetic subjects (P > 0.05). However, PPD significantly increased over time in both groups (P = 0.016 in the healthy group and P = 0.007 in diabetic one) with no significant difference between them.
Gomez-Moreno et al evaluated PPD, BOP, and marginal bone loss in different diabetic groups with variable levels of HbA1c. They showed that PPD and marginal bone loss increased with an increase in blood glucose level, but the differences among the groups were not significant. They observed a significant increase in BOP by an increase in level of HbA1c (13), which was different from our findings and may have been due to the smaller sample size in our study. Oates et al evaluated 177 patients and a total of 234 implants, but failed to find a significant correlation between implant survival and high level of HbA1c in their one-year follow-up. They only reported that primary bone healing and implant stability were correlated with the level of HbA1c (20).
Aguilar-Salvatierra et al followed up the patients for 2 years and found a significant correlation between implant survival and HbA1c level. They reported that an increase in blood glucose level significantly increased the marginal bone loss and BOP, which was inconsistent with our findings, and may have been attributed to their larger sample size or difference in culture and, subsequently, in oral hygiene. Alternatively, this difference may have been due to different implant placement protocols. They also found lower PPD in HbA1c ≤6 group compared with HbA1c >6 group. Moreover, PPD increased in all groups but no significant differences were observed among the groups in the 1-month, 1-year, and 2-year follow-ups. Their results were in line with our findings, although they had a larger sample size and longer follow-up period. Al-Sowygh et al followed up 93 patients for 62 months and found a significant difference in PPD between diabetic and non-diabetic patients. Thus, it may have been stated that achieving a significant difference in PPD between diabetic and non-diabetic patients required a follow-up period longer than 2 years (21). Al-Sowygh et al evaluated BOP, PPD, and bone loss and reported a significant difference between diabetic and non-diabetic individuals. The difference between their study results and ours may have been due to the time of measurements since they had measured the parameters after a minimum of 12 months following implant placement in all groups, while the parameters in this study were measured after one and six month(s). Also, they divided the diabetic patients into three groups with HbA1c levels of 6-8, 8-10 and >10 and reported the results separately for each group. For instance, patients with HbA1c levels of 8-10 and >10 showed a highly significant difference in the measured parameters, while all patients with HbA1c > 6 were considered as diabetic in this study.
Poor blood glucose control has been a contraindication for dental implant placement for a long time (22). Currently, precise blood sugar control is a critical goal to minimize the risk of DM complications in type II diabetic patients (23). Therefore, patients with uncontrolled DM are not considered as good candidates for implant placement (22). Available evidence indicates that the results from recent studies on the effects of hyperglycemia on implant treatment are inconclusive (24). To put it another way, these effects have not been well-elucidated yet. Moreover, patients with properly controlled DM can benefit from implant treatment given the fact that they follow a strict nutritional regimen.
The safety and high success rate of dental implant treatments have been previously documented. However, peri-implant mucositis and peri-implantitis can compromise the success of dental implants. These conditions are aggravated in patients with underlying systemic conditions such as DM (25,26). In this study, no significant difference was found between diabetic and non-diabetic patients regarding BOP, bone loss, and PPD in one and six month(s) after implant placement. Taking into consideration the results from other studies with longer follow-up periods and more precise grouping of diabetic patients based on HbA1c level, however, it was recommended that dental implant candidates should measure their HbA1c level preoperatively; and if its level was <8%, they could undergo dental implant placement given the fact that they followed a strict nutritional regime under constant monitoring of an endocrinologist (17). It was also recommended that further studies with larger sample size and longer follow-up period be carried out to investigate the subject more thoroughly.
Conclusions
Given the limitations of this study, no significant difference was found between non-diabetic and diabetic patients in terms of PPD, BOP and marginal bone loss with hbA1c<9 during six months follow up.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Ethical Statement
This study was approved by the ethics committee of Shahid Sadoughi University of Medical Science, IR.SSU.REC.1397.104.
Authors’ Contribution
Study concept and design: AK; acquisition of data, AF; analysis and interpretation of data, HP; drafting of the manuscript, SS; critical revision of the manuscript for im-portant intellectual content, AK, SS; statistical analysis, HP; administrative, technical, and materialsupport, AF; study supervision, : AK, AF.
Funding
The authors declare that there was no external source of funding for the present study.
Acknowledgments
This study was based on a thesis submitted to the school of Dentistry, Shahid Sadoughi University of Medical Sciences, in partial fulfilment of the requirement for the DDS/MSC degree. This study was supported by Shahid Sadoughi University of Medical Sciences (Research Grant #986011).
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37 Suppl 1:S81-90. doi: 10.2337/dc14-S081 [Crossref] [ Google Scholar]
- Ghane’i R, Golkar F. Foot care in depressed and non-depressed diabetic patients. Mod Care J 2013; 10(2):124-31. [ Google Scholar]
- Shojaeizadeh DA, Estebsari F, Aezam K, Batebi A, Mostafaei DA. Comparison of diabetes type 2 patients life style effective factors with that of healthy people. The Journal of Shahid Sadoughi University of Medical Sciences 2008; 16(2):71-9. [ Google Scholar]
- Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res 2012; 2012:789174. doi: 10.1155/2012/789174 [Crossref] [ Google Scholar]
- Maroufizadeh S, Almasi-Hashiani A, Hosseini M, Sepidarkish M, Omani Samani R. Prevalence of diabetic retinopathy in Iran: a systematic review and Meta-analysis. Int J Ophthalmol 2017; 10(5):782-9. doi: 10.18240/ijo.2017.05.21 [Crossref] [ Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131):837-53. [ Google Scholar]
- Ryan ME. Diagnostic & therapeutic strategies for the management of the diabetic patient. Compend Contin Educ Dent 2008; 29(1):32-8, 40. [ Google Scholar]
- Lalla RV, D’Ambrosio JA. Dental management considerations for the patient with diabetes mellitus. J Am Dent Assoc 2001; 132(10):1425-32. doi: 10.14219/jada.archive.2001.0059 [Crossref] [ Google Scholar]
- Tan WC, Tay FB, Lim LP. Diabetes as a risk factor for periodontal disease: current status and future considerations. Ann Acad Med Singap 2006; 35(8):571-81. [ Google Scholar]
- Marchand F, Raskin A, Dionnes-Hornes A, Barry T, Dubois N, Valéro R, Vialettes B. Dental implants and diabetes: conditions for success. Diabetes & metabolism 2012 Feb 1; 38(1):14-9. [ Google Scholar]
- Lindh T, Gunne J, Tillberg A, Molin M. A meta-analysis of implants in partial edentulism. Clin Oral Implants Res 1998; 9(2):80-90. doi: 10.1034/j.1600-0501.1998.090203.x [Crossref] [ Google Scholar]
- Mellado-Valero A, Ferrer García JC, Herrera Ballester A, Labaig Rueda C. Effects of diabetes on the osseointegration of dental implants. Med Oral Patol Oral Cir Bucal 2007; 12(1):E38-43. [ Google Scholar]
- Gómez-Moreno G, Aguilar-Salvatierra A, Rubio Roldán J, Guardia J, Gargallo J, Calvo-Guirado JL. Peri-implant evaluation in type 2 diabetes mellitus patients: a 3-year study. Clin Oral Implants Res 2015; 26(9):1031-5. doi: 10.1111/clr.12391 [Crossref] [ Google Scholar]
- Eskow CC, Oates TW. Dental implant survival and complication rate over 2 years for individuals with poorly controlled type 2 diabetes mellitus. Clin Implant Dent Relat Res 2017; 19(3):423-31. doi: 10.1111/cid.12465 [Crossref] [ Google Scholar]
- Monje A, Catena A, Borgnakke WS. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: Systematic review and meta-analysis. J Clin Periodontol 2017; 44(6):636-48. doi: 10.1111/jcpe.12724 [Crossref] [ Google Scholar]
- Ormianer Z, Block J, Matalon S, Kohen J. The effect of moderately controlled type 2 diabetes on dental implant survival and peri-implant bone loss: a long-term retrospective study. Int J Oral Maxillofac Implants 2018; 33(2):389-94. doi: 10.11607/jomi.5838 [Crossref] [ Google Scholar]
- Aguilar-Salvatierra A, Calvo-Guirado JL, González-Jaranay M, Moreu G, Delgado-Ruiz RA, Gómez-Moreno G. Peri-implant evaluation of immediately loaded implants placed in esthetic zone in patients with diabetes mellitus type 2: a two-year study. Clin Oral Implants Res 2016; 27(2):156-61. doi: 10.1111/clr.12552 [Crossref] [ Google Scholar]
- Winter WE, Signorino MR. Diabetes Mellitus: Pathophysiology, Etiologies, Complications, Management, and Laboratory Evaluation: Special Topics in Diagnostic Testing. Washington, DC: AACC Press; 2003.
- Little J, Falace D, Miller C, Rhodus N. Dental Management of the Medically Compromised Patients. 7th ed. St. Louis: Mosby; 2008. p. 433-64.
- Oates TW Jr, Galloway P, Alexander P, Vargas Green A, Huynh-Ba G, Feine J. The effects of elevated hemoglobin A(1c) in patients with type 2 diabetes mellitus on dental implants: survival and stability at one year. J Am Dent Assoc 2014; 145(12):1218-26. doi: 10.14219/jada.2014.93 [Crossref] [ Google Scholar]
- Al-Sowygh ZH, Ghani SMA, Sergis K, Vohra F, Akram Z. Peri-implant conditions and levels of advanced glycation end products among patients with different glycemic control. Clin Implant Dent Relat Res 2018; 20(3):345-51. doi: 10.1111/cid.12584 [Crossref] [ Google Scholar]
- Javed F, Romanos GE. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: a systematic literature review. J Periodontol 2009; 80(11):1719-30. doi: 10.1902/jop.2009.090283 [Crossref] [ Google Scholar]
- American Diabetes Association. The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes 1996; 45(10):1289-98. [ Google Scholar]
- Oates TW, Huynh-Ba G, Vargas A, Alexander P, Feine J. A critical review of diabetes, glycemic control, and dental implant therapy. Clin Oral Implants Res 2013; 24(2):117-27. doi: 10.1111/j.1600-0501.2011.02374.x [Crossref] [ Google Scholar]
- Alsaadi G, Quirynen M, Komárek A, van Steenberghe D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol 2007; 34(7):610-7. doi: 10.1111/j.1600-051X.2007.01077.x [Crossref] [ Google Scholar]
- Kotsovilis S, Karoussis IK, Fourmousis I. A comprehensive and critical review of dental implant placement in diabetic animals and patients. Clin Oral Implants Res 2006; 17(5):587-99. doi: 10.1111/j.1600-0501.2005.01245.x [Crossref] [ Google Scholar]