Avicenna J Dent Res. 15(2):59-62.
doi: 10.34172/ajdr.2023.433
Original Article
A Histochemical Comparison of Feulgen and Papanicolaou Stains in Demonstrating Cytotoxic and Genotoxic Effects of Cigarette Smoking on Human Buccal Mucosa Cells
Iman Yarmohammadi 1  , Noushin Jalayer Naderi 2, *
, Noushin Jalayer Naderi 2, * 
Author information:
1Graduated from Faculty of Dentistry, Shahed University, Tehran, Iran
2Department of Oral and Maxillofacial Pathology, Faculty of Dentistry, Shahed University, Tehran, IranDepartment of Oral and Maxillofacial Pathology, Faculty of Dentistry, Shahed University, Tehran, Iran
Abstract
Background: Different histochemical stains have been applied to demonstrate the cytotoxic and genotoxic effects of cigarette smoking on cells. Feulgen and Papanicolaou were the most popular stains to demonstrate nuclear abnormalities. The aim of this study was to compare Feulgen and Papanicolaou stains in demonstrating the cytotoxic and genotoxic effects of cigarette smoking on exfoliated oral mucosa cells.
Methods: A total of 31 cigarette smokers and 15 non-smokers were included in this case-control study. Using a wooden spatula, two samples were taken from each participant. The samples from the left buccal mucosa were stained with Feulgen and the right mucosa with Papanicolaou. The mean number of micronuclei and the number of cells with pyknosis, karyorrhexis, and karyolysis were determined on Feulgen and Papanicolaou-stained slides. The number of counted cells with pyknosis, karyorrhexis, and karyolysis in 1000 cells/subject was recorded. The mean number of micronuclei was determined by the number of counted micronuclei per 1000 cells per subject.
Results: The number of micronuclei was not significantly different between Feulgen and Papanicolaou stained samples (P=0.27). Demonstration of karyolysis (P=0.73) and karyorrhexis (P=0.24) was not significantly different between Feulgen and Papanicolaou staining methods. The Feulgen was significantly more effective in demonstrating pyknosis compared to Papanicolaou (P=0.02).
Conclusions: Feulgen and Papanicolaou stains had similar effectiveness in demonstrating DNA alterations (micronucleus) and cellular death features (karyorrhexis and karyolysis). Feulgen was preferable to display pyknosis than Papanicolaou.
Keywords: Assay, Buccal, Cytotoxicity, Micronucleus
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Yarmohammadi I, Jalayer Naderi N. A histochemical comparison of feulgen and papanicolaou stains in demonstrating cytotoxic and genotoxic effects of cigarette smoking on human buccal mucosa cells. Avicenna J Dent Res. 2023; 15(2):59-62. doi:10.34172/ajdr.2023.433
Background
Nuclear changes in a cytotoxic event lead to apoptosis. Pyknosis, karyorrhexis, and karyolysis are nuclear features of apoptosis (1). In a genotoxic event, a carcinogenic agent creates micronuclei. A micronucleus is a separated part of the nucleus that arises from chromosomal fragments (2). Evaluation of DNA alterations (micronucleus count), cellular proliferation (basal cells), and cellular death features (pyknosis, karyorrhexis, and karyolysis) are reliable biomarkers to examine the nucleus condition. These biomarkers are functional indicators of biological changes that determine the susceptibility of individuals to cancer (3).
Biomonitoring of genotoxic and cytotoxic agents is a simple method for detecting harmful and carcinogenic effects. Comparing different findings of a screening system requires the determination of a standard method. The first step in the development of a standardized approach is the uniformization of materials. Different histochemical stains have been applied to demonstrate the cytotoxic and genotoxic effects of cigarette smoking on cells. Feulgen and Papanicolaou were the most popular stains to demonstrate nuclear abnormalities (4-6). It has been shown that an applied stain can affect the results and create false positive /false negative conclusions (7).
Evaluation of the genotoxic and cytotoxic effects of agents is of great importance in screening patients who are at higher risk of cancer. Little attention has been paid to the effect of different histochemical stains on the detection of genetic variations and nuclear features of apoptosis in smokers. The aim of this study was to compare Feulgen and Papanicolaou stains in demonstrating the cytotoxic and genotoxic effects of cigarette smoking on exfoliated oral mucosa cells.
Materials and Methods
Thepresentstudy was a case-control study. The sampling was conducted in the Faculty of Dentistry, Shahed University, from October to December 2017. The sample size was determined to be 31 subjects in cases (smokers) and 15 in controls (nonsmokers) assuming a power of 0.9 with 95% confidence interval. All enrolled subjects were 20-to-50-year-old males from Tehran, Iran. Suffering from oral and systemic diseases and exposure to dental radiography in the last 6 months were exclusion criteria. Drugs and/or alcohol consumers, waterpipe smokers, industrial workers, and farmers who worked with pesticides did not enter the study. The smokers who quit smoking in the past three years and smoked for less than three years were excluded. In controls, participants with a history of smoking did not enter the study (8). The number of smoked cigarettes per year (Pack years) was recorded for all subjects (4). According to the formula, cases with the rate of smoking from 200 to 500 Pack years were included in the study.
All participants signed an informed consent before sampling. The name of the subjects was not used during the study. The basic information including age and pack years of smoking was registered and coded. All subjects were examined and sampled by a dental student. Two samples were taken from each participant. The obtained samples from the left buccal mucosa were stained with Feulgen and the right mucosa with Papanicolaou. After rinsing the mouth twice with water, the buccal mucosa cells were collected using a sterile disposable plastic spatula. The exfoliated buccal cells were spread on the glass slides and then fixed with Carnoy’s fixative (methanol and glacial acetic acid in a ratio of 3:1) for 30 minutes and dried at room temperature.
Obtained slides were prepared and stained by a laboratory technician. The modified method of Thomas et al was used for preparing Feulgen-stained slides. Feulgen staining was completed by dipping the slides in 1 N HCL at 60℃ for 30 minutes, rinsing them in the distilled water for 3 minutes, placing them in Schiff’s reagent for 60 minutes and normal saline for 10 minutes, placing them in 5% sodium metabisulfite solution 3 times, and rinsing them with tap water. Finally, slides were stained with 1% light green for 15 minutes, rinsed with tap water, dried, and mounted (9).
The conventional Papanicolaou staining method was used. The fixed slides were immersed in absolute, 70%, and 50% alcohol for 2 minutes in each section. After rinsing with water, the slides were stained with hematoxylin for 4 minutes, rinsed with water, placed in acid alcohol for 5 seconds, dehydrated with absolute alcohol, and stained with orange G for 10 seconds. Using the EA 50 Pap reagent, absolute alcohol, xylene and mounting were the final steps.
The cytotoxicity of cigarette was evaluated by the number of cells with pyknosis, karyorrhexis, and karyolysis. The method developed by Tolbert et al was used for evaluating the cells with pyknosis, karyorrhexis, and karyolysis. The cells with aggregated chromatin, disintegrated nucleus, and dissolved nucleus were considered pyknosis, karyorrhexis, and karyolysis, respectively (10) (Figure 1). Genotoxicity was examined by counting the micronuclei. The cytoplasmic structure with 1/3 to 1/5 size of the nucleus and nuclear stain was considered micronucleus (11). To evaluate the cytotoxic and genotoxic changes, the overlapped cells were not counted. The cells with distinctive cellular boundaries were included in the examination. The number of counted cells with pyknosis, karyorrhexis, and karyolysis in 1000 cells/subject was determined (4). The mean number of micronucleus was determined by the number of counted micronuclei per 1000 cells per subject (12).
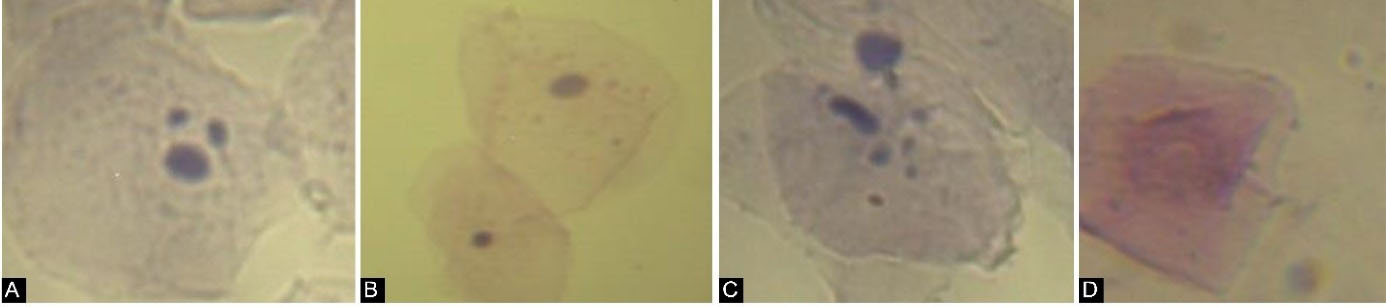
Figure 1.
DNA Alterations (Micronucleus) and Cellular Death Features (Pyknosis, Karyorrhexis, and Karyolysis) in Human Buccal Mucosa Cells. (A) Micronucleus (Feulgen staining, 400), (B) Pyknosis (arrow) (Papanicolaou staining, 400), (C) Karyorrhexis (Feulgen staining, 400), (D) Karyolysis (Papanicolaou staining, 400)
.
DNA Alterations (Micronucleus) and Cellular Death Features (Pyknosis, Karyorrhexis, and Karyolysis) in Human Buccal Mucosa Cells. (A) Micronucleus (Feulgen staining, 400), (B) Pyknosis (arrow) (Papanicolaou staining, 400), (C) Karyorrhexis (Feulgen staining, 400), (D) Karyolysis (Papanicolaou staining, 400)
The counts were completed using an optical microscope (Olympus BX40) equipped with a digital camera (Sony ExwaveHAD, Model No. SSC-DC58AP; Tokyo, Japan) by an experienced oral pathologist. The cells were evaluated blind at 1000 magnification (10 ocular and 100 objective lenses). Data were analyzed using t test and Levene tests at P ≤ 0.05. The data were presented as the as mean ± SD. The SPSS version 20.0 (IBM Company, Chicago, IL, USA) was employed to analyze the data.
Results
In nonsmokers, the age ranged from 20 to 48 years with a mean of 28.9 ± 9.3 years. In the smokers group, the age ranged from 21 to 50 years with a mean of 34.1 ± 10.8 years.
The mean number of pyknosis, karyorrhexis, and karyolysis in smokers was 8.22 ± 4.9, 0.35 ± 0.65, and 2.98 ± 3.3, respectively. The mean number of pyknosis, karyorrhexis, and karyolysis in nonsmokers was 4.4 ± 2.25, 0.03 ± 0.18, and 0.63 ± 0.92, respectively (Table 1).
Table 1.
The Mean Number of Micronuclei (Expressing Genotoxicity) and Pyknosis, Karyorrhexis, and Karyolysis Counts (Representing Cytotoxicity) Using Feulgen and Papanicolaou Staining in Smokers and Non-smokers
|
|
Feulgen
|
Papanicolaou
|
|
Pyknosis
|
Karyorrhexis
|
Karyolysis
|
Micronucleus
|
Pyknosis
|
Karyorrhexis
|
Karyolysis
|
Micronucleus
|
| Smokers |
9.61 ± 5.1 |
0.45 ± 0.77 |
2.84 ± 0.57 |
6.06 ± 5.39 |
6.84 ± 4.4 |
0.25 ± 0.51 |
3.13 ± 0.62 |
8.35 ± 10.14 |
| Non-smokers |
5 ± 2.69 |
0 |
1.1 ± 1.06 |
3.6 ± 3.6 |
3.8 ± 1.5 |
0.06 ± 0.2 |
0.13 ± 0.3 |
3.8 ± 4.07 |
The results of t test revealed a significant difference between smokers and non-smokers in terms of cytotoxicity (P = 0). Levene test indicated that cytotoxicity in terms of pyknosis (P = 0), karyorrhexis (P = 0.001), and karyolysis (P = 0) in smokers was significantly higher compared to non-smokers. The micronucleus counts in smokers and non-smokers were 7.21 ± 8.13 and 3.7 ± 3.78, respectively. The genotoxicity was significantly higher in smokers than in non-smokers (P = 0.006).
The results of the t test revealed that the demonstration of micronuclei was not significantly different between Feulgen and Papanicolaou (P = 0.27). Demonstration of karyolysis (P = 0.73) and karyorrhexis (P = 0.24) was not significantly different between Feulgen and Papanicolaou staining methods. The Feulgen stain was significantly more successful in demonstrating pyknosis (P = 0.02) compared to Papanicolaou.
Discussion
The study shows that Feulgen and Papanicolaou stains were similar in demonstrating DNA alterations (micronucleus) and cellular death features (karyorrhexis and karyolysis). The Feulgen technique was more effective in demonstrating pyknosis compared to Papanicolaou.
Cigarette smoking has genotoxic and cytotoxic effects on human buccal mucosa cells. Researchers have shown the increasing rate of micronucleus (6-14) and apoptosis (4-15) in cigarette smokers. This is compatible with the present findings.
The staining method is an important tool for determining the nucleus abnormalities. The false positive/false negative conclusions are routine outcomes of unsuitable stains (7). The protocol developed by Stich et al to assess the genotoxic effect of carcinogens on exfoliated buccal mucosa cells is still extensively used (16). To demonstrate the nuclear features based on this protocol, different staining methods have been used. Feulgen (4,17,18) and Papanicolaou (12,19,20) are the most popular stains used in displaying the nucleus changes. Basically, DNA-specific stains have more appropriate results in demonstrating nuclear changes. Feulgen is a DNA-specific stain and Papanicolaou is a nonspecific histochemical stain. Both Feulgen and Papanicolaou are popular in cytologic studies, but which one can be better in demonstrating the cytotoxic/genotoxic effects of agents?
Fulgen and Papanicolaou stains were similar in demonstrating DNA alterations and cellular death features. The only exception was the representation of pyknotic structures. The finding is not compatible with the study of Kumar et al who showed that the number of micronuclei in Papanicolaou-stained slides was significantly higher compared to Feulgen-stained samples (21).
The results are compatible with previous studies which showed the overestimation of micronuclei count with non-DNA-specific stains such as Giemsa-based stains (7). Nonspecific DNA stains in evaluating genotoxic changes may lead to false-positive results. This finding was in agreement with results obtained by Casartelli et al and Holland et al (22,23).
A recent study by Kohli et al showed better results for Papanicolaou stain than May-Grünwald Giemsa stain and Feulgen stain in the evaluation of micronuclei (24). This is not in agreement with the present study.
Binucleation, condensed chromatins, karyorrhexis, and karyolysis cause misinterpretation of micronuclei count with DNA nonspecific stained preparations (7,24). Abnormal changes in the nucleus are not specific to damaged cells. Nuclear anomalies can also be observed in normal undamaged cells. Contradictory results may be due to not considering all cellular changes at the same time.
To compare the Papanicolaou and Feulgen stains in terms of these changes, control samples were selected from non-smokers. Although the rate of nuclear changes in smokers was higher compared to non-smokers, there were no differences between the two stains in demonstrating the nuclear features.
Accurate sampling, appropriate fixation, and good stained sections are important subjects in evaluating micronuclei. For proper evaluation of samples, the use of standard criteria to identify cellular abnormalities is necessary. To obtain more reliable and valid results, the protocol developed by Tolbert et al was used to evaluate microscopic abnormalities (10). Simple laboratory procedure and the lower cost of Papanicolaou staining method in comparison with Feulgen are important reasons for choosing Papanicolaou. Feulgen staining technique is time-consuming and needs an experienced technician to complete. Based on our experience, selecting the Papanicolaou or Feulgen staining methods is the second important step in cellular evaluation. The most important issue to evaluate cellular changes is the use of standard criteria.
Based on the present results, simultaneous evaluation of DNA alterations (micronucleus) and cellular death features (karyorrhexis, karyolysis, and pyknosis) is suggested. Standardization of definitions of DNA alterations is intensely recommended in future studies.
Conclusions
Feulgen and Papanicolaou staining methods had similar effectiveness in demonstrating DNA alterations (micronucleus) and cellular death features (karyorrhexis and karyolysis). The Feulgen staining method was preferable to display pyknosis compared to Papanicolaou.
Authors’ Contribution
Conceptualization: Noushin Jalayer Naderi.
Data curation: Iman Yarmohammadi, Noushin Jalayer Naderi.
Formal analysis: Noushin Jalayer Naderi.
Investigation: Iman Yarmohammadi, Noushin Jalayer Naderi.
Methodology: Noushin Jalayer Naderi.
Project administration: Noushin Jalayer Naderi.
Supervision: Noushin Jalayer Naderi.
Validation: Noushin Jalayer Naderi.
Visualization: Iman Yarmohammadi, Noushin Jalayer Naderi.
Writing–original draft: Iman Yarmohammadi, Noushin Jalayer Naderi.
Writing–review & editing: Iman Yarmohammadi, Noushin Jalayer Naderi.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The study was approved by the Ethics Committee of Shahed University (IR.Shahed.REC.1395.217).
References
- Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: methods development. Mutat Res 1992; 271(1):69-77. doi: 10.1016/0165-1161(92)90033-i [Crossref] [ Google Scholar]
- Ramirez A, Saldanha PH. Micronucleus investigation of alcoholic patients with oral carcinomas. Genet Mol Res 2002; 1(3):246-60. [ Google Scholar]
- Çelik A, Yildirim S, Ekinci SY, Taşdelen B. Bio-monitoring for the genotoxic assessment in road construction workers as determined by the buccal micronucleus cytome assay. Ecotoxicol Environ Saf 2013; 92:265-70. doi: 10.1016/j.ecoenv.2013.01.030 [Crossref] [ Google Scholar]
- Jalayer Naderi N, Pour Pasha M. Comparison of cytotoxic effect of cigarette and waterpipe smoking on human buccal mucosa. Int J Prev Med 2017; 8:98. doi: 10.4103/ijpvm.IJPVM_62_17 [Crossref] [ Google Scholar]
- Bansal H, Sandhu VS, Bhandari R, Sharma D. Evaluation of micronuclei in tobacco users: a study in Punjabi population. Contemp Clin Dent 2012; 3(2):184-7. doi: 10.4103/0976-237x.96825 [Crossref] [ Google Scholar]
- Motgi AA, Chavan MS, Diwan NN, Chowdhery A, Channe PP, Shete MV. Assessment of cytogenic damage in the form of micronuclei in oral epithelial cells in patients using smokeless and smoked form of tobacco and non-tobacco users and its relevance for oral cancer. J Cancer Res Ther 2014; 10(1):165-70. doi: 10.4103/0973-1482.131454 [Crossref] [ Google Scholar]
- Nersesyan A, Kundi M, Atefie K, Schulte-Hermann R, Knasmüller S. Effect of staining procedures on the results of micronucleus assays with exfoliated oral mucosa cells. Cancer Epidemiol Biomarkers Prev 2006; 15(10):1835-40. doi: 10.1158/1055-9965.epi-06-0248 [Crossref] [ Google Scholar]
- Kumar V, Faizuddin M. Effect of smoking on gingival microvasculature: a histological study. J Indian Soc Periodontol 2011; 15(4):344-8. doi: 10.4103/0972-124x.92566 [Crossref] [ Google Scholar]
- Thomas P, Hecker J, Faunt J, Fenech M. Buccal micronucleus cytome biomarkers may be associated with Alzheimer’s disease. Mutagenesis 2007; 22(6):371-9. doi: 10.1093/mutage/gem029 [Crossref] [ Google Scholar]
- Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: a field test in snuff users. Am J Epidemiol 1991; 134(8):840-50. doi: 10.1093/oxfordjournals.aje.a116159 [Crossref] [ Google Scholar]
- Sarto F, Finotto S, Giacomelli L, Mazzotti D, Tomanin R, Levis AG. The micronucleus assay in exfoliated cells of the human buccal mucosa. Mutagenesis 1987; 2(1):11-7. doi: 10.1093/mutage/2.1.11 [Crossref] [ Google Scholar]
- El-Setouhy M, Loffredo CA, Radwan G, Abdel Rahman R, Mahfouz E, Israel E. Genotoxic effects of waterpipe smoking on the buccal mucosa cells. Mutat Res 2008; 655(1-2):36-40. doi: 10.1016/j.mrgentox.2008.06.014 [Crossref] [ Google Scholar]
- de Geus JL, Wambier LM, Bortoluzzi MC, Loguercio AD, Kossatz S, Reis A. Does smoking habit increase the micronuclei frequency in the oral mucosa of adults compared to non-smokers? A systematic review and meta-analysis. Clin Oral Investig 2018; 22(1):81-91. doi: 10.1007/s00784-017-2246-4 [Crossref] [ Google Scholar]
- Oliveira LU, Lima CF, Salgado MA, Balducci I, Almeida JD. Comparative study of oral mucosa micronuclei in smokers and alcoholic smokers. Anal Quant Cytol Histol 2012; 34(1):9-14. [ Google Scholar]
- Haveric A, Haveric S, Ibrulj S. Micronuclei frequencies in peripheral blood and buccal exfoliated cells of young smokers and non-smokers. Toxicol Mech Methods 2010; 20(5):260-6. doi: 10.3109/15376516.2010.482962 [Crossref] [ Google Scholar]
- Stich HF, Stich W, Parida BB. Elevated frequency of micronucleated cells in the buccal mucosa of individuals at high risk for oral cancer: betel quid chewers. Cancer Lett 1982; 17(2):125-34. doi: 10.1016/0304-3835(82)90024-6 [Crossref] [ Google Scholar]
- Nandini DB, Subramanyam RV. Nuclear features in oral squamous cell carcinoma: a computer-assisted microscopic study. J Oral Maxillofac Pathol 2011; 15(2):177-81. doi: 10.4103/0973-029x.84488 [Crossref] [ Google Scholar]
- Keyes M, Macaulay C, Hayes M, Korbelik J, Morris WJ, Palcic B. DNA ploidy measured on archived pretreatment biopsy material may correlate with prostate-specific antigen recurrence after prostate brachytherapy. Int J Radiat Oncol Biol Phys 2013; 86(5):829-34. doi: 10.1016/j.ijrobp.2013.04.011 [Crossref] [ Google Scholar]
- Seifi S, Feizi F, Mehdizadeh M, Khafri S, Ahmadi B. Evaluation of cytological alterations of oral mucosa in smokers and waterpipe users. Cell J 2014; 15(4):302-9. [ Google Scholar]
- Giridharan J, Natarajan UA, Kotasthane DS. Predictive value of micronucleus count in cervical smears of normal, infective inflammatory & intraepithelial neoplasia pathology in perimenopausal women. Int J Sci Res 2014; 3(10):1571-4. [ Google Scholar]
- Kumar M, Prasad UC, Chandolia B, Manjunath SM, Basu S, Verma S. Can feulgen stain be a reliable biomarker over PAP stain for estimation of micronuclei score?. J Clin Diagn Res 2016; 10(10):ZC07-ZC11. doi: 10.7860/jcdr/2016/18859.8630 [Crossref] [ Google Scholar]
- Casartelli G, Monteghirfo S, De Ferrari M, Bonatti S, Scala M, Toma S. Staining of micronuclei in squamous epithelial cells of human oral mucosa. Anal Quant Cytol Histol 1997; 19(6):475-81. [ Google Scholar]
- Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmueller S. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat Res 2008; 659(1-2):93-108. doi: 10.1016/j.mrrev.2008.03.007 [Crossref] [ Google Scholar]
- Kohli M, Ahuja P, Mehendiratta M, Sharma M, Dutta J. Micronucleus assay: an early diagnostic tool to assess genotoxic changes in patients with tobacco use, oral leukoplakia and oral submucous fibrosis. J Clin Diagn Res 2017; 11(9):ZC28-ZC32. doi: 10.7860/jcdr/2017/27711.10567 [Crossref] [ Google Scholar]