Avicenna J Dent Res. 13(1):28-37.
doi: 10.34172/ajdr.2021.06
Review Article
The Effect of Statins on Orthodontic Tooth Movements: A Meta-analysis of Animal Studies
Zohreh Afshari 1  , Shabnam Tahamtan 2, *
, Shabnam Tahamtan 2, *  , Farinaz Shirban 2
, Farinaz Shirban 2 
Author information:
1Postgraduate Student, Department of Periodontics, Dental Students Research Committee, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran.
2Assistant Professor, Dental Research Center, Department of Orthodontics, Dental Research Institute, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran.
Abstract
Background: Statins are effective therapeutic agents for the treatment of cardiovascular diseases. Their favorable effects on various aspects of oral health including promising effects on bone metabolism and pleiotropic impacts such as anti-inflammatory properties made these drugs a current area of interest in the field of orthodontics. Therefore, the aim of this study was to evaluate the effects of statins on orthodontic tooth movement (OTM) in animals undergoing orthodontic treatments.
Methods: Several databases were comprehensively searched for studies measuring the effects of statins on the OTM up to January 2020, including MEDLINE, ISI Web of Science, EMBASE, Scopus, and Cochrane. Animal studies evaluating the effects of statins on tooth movements in animals undergoing orthodontic treatments were selected based on the PICO model.Study selection, data extraction, risk of bias, and study quality assessment were independently performed by two reviewers. Finally, the data were analyzed using random-effects meta-analysis and the mean difference (MD) was used for comparing the outcome measures.
Results: Three randomized trials were finally included in this meta-analysis. According to the Systematic Review Centre for Laboratory animal Experimentation Tool, all the included studies had at least one domain at a high risk of bias. The amount of the OTM was insignificantly lower in the statin group (MD = 0.134 mm, %95 confidence interval = -0.020-0.288, P>0.05).
Conclusions: Due to the low quality and methodological inconsistencies among the included studies, conclusive confirmation regarding the effect of statins on the OTM remains debatable. Trail Registration: The protocol of this study was registered on PROSPERO (https://www.crd.york.ac.uk/PROSPERO/) with the ID # CRD42020164155.
Keywords: Statin, Orthodontic tooth movement, Orthodontic treatment, Tooth movement
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Afshari Z, Tahamtan S, Shirban F. The effect of statins on orthodontic tooth movements: A Meta-analysis of Animal Studies. Avicenna J Dent Res. 2021;13(1):28-37. doi: 10.34172/ajdr.2020.06.
Background
Nowadays, the prevalence of obesity-related diseases such as hyperlipidemia has increased, especially in adults and causes atherosclerosis and other coronary diseases. Lipid-lowering medications such as the statin family of drugs are mostly used by these patients. Clinical trials have shown that statins are well-tolerated in adult and younger populations (1-3).
The statin family of drugs is a safe therapeutic agent for the treatment of arteriosclerotic cardiovascular disease. They act as potential inhibitors of 3-hydroxy-3-methylglutaryl reductase A (HMG-CoA), which is a rate-limiting enzyme in the mevalonate pathway of cholesterol biosynthesis, thus preventing the synthesis of cholesterol in the liver and reducing the levels of blood cholesterol and triglycerides (4). In addition to their cholesterol-lowering effects, it is reported that statins have several promising effects on human health, including pleiotropic effects, improvement of endothelial function, and anti-inflammatory, antioxidant, and immunomodulatory effects (5-13). It has been clarified that statins affect bone metabolism in different ways. They stimulate the osteoblastic differentiation of bone marrow stem cells through the increased gene expression of bone morphogenic protein-2 and angiogenesis. Statins may also stimulate bone formation by preventing osteoblastic apoptosis (14-18). Furthermore, statins inhibit bone resorption through suppressing osteoclastogenesis (19,20). Thus, they could influence orthodontic tooth movements (OTM) and orthodontic relapse in adult patients by their stimulatory effects on bone formation and pleiotropic effects such as anti-inflammatory and immunomodulatory ones (21). Factors affecting the remodeling process will influence OTM (22). Pharmacological factors could potentially affect tooth movements either for reducing (when anchorage strengthening is desirable) or increasing the movement (23-26). Various experimental trials evaluated the effects of pharmacologic factors on the periodontal responses of the OTM. Various animal studies have proved the stimulatory effects of statins on bone formation during its use by different carriers (9,15,27-35). Statins are one of the most commonly prescribed therapeutic agents for the prevention of cardiovascular diseases, thus their plausible effect of arresting tooth movements in adult patients could justify their relevance in orthodontic practice. Considering these facts, the present study aimed to systematically review the efficacy of statin delivery in orthodontic movements.
Materials and Methods
Protocol
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines (36). The protocol of this study was registered on PROSPERO (https://www.crd.york.ac.uk/PROSPERO/) with the ID # CRD42020164155.
Focus Question
The present systematic review and meta-analysis sought to assess the null hypothesis that “there would be no difference in the amount of the OTM by the administration of statins”. The focused question according to the PICO format (i.e., Population, Intervention, Control group, and Outcome) was “whether there is a significant decrease in the OTM of statin receivers compared to the control group in animal models ”. Population (P) indicated animals who underwent orthodontic treatments; Interventions (I) represented orthodontic treatments with statin administration. Finally, control intervention (C) included orthodontic treatments without adjunct statin administration and outcome measured (O) denoted the amount of the OTM.
Search Strategy
An electronic search was performed until 1st January 2020 to acquire potentially eligible studies with no time or language restrictions in several electronic bibliographic databases such as PubMed, EMBASE, Web of Science, Scopus, and Cochrane. The search strategies of each database are presented in Table 1. The reference part of the retrieved full-text articles (cross-referencing) was also searched for further papers. Non-English translatable papers were included in this study.
Table 1.
Databases, Applied Search Strategy and Numbers of Retrieved Studies
|
Database of Published Trials
|
Applied Search Strategy
|
Hits
|
MEDLINE searched via PubMed on 1 January 2020 via
www.ncbi.nlm.nih.gov/sites |
((orthodontic[All Fields] OR ("tooth movement techniques"[MeSH Terms] OR ("tooth"[All Fields] AND "movement"[All Fields] AND "techniques"[All Fields]) OR "tooth movement techniques"[All Fields] OR ("tooth"[All Fields] AND "movement"[All Fields]) OR "tooth movement"[All Fields])) AND ((("simvastatin"[MeSH Terms] OR "fluvastatin"[MeSH Terms]) OR ("hydroxymethylglutaryl-coa reductase inhibitors"[Pharmacological Action] OR "hydroxymethylglutaryl-coa reductase inhibitors"[MeSH Terms] OR ("hydroxymethylglutaryl-coa"[All Fields] AND "reductase"[All Fields] AND "inhibitors"[All Fields]) OR "hydroxymethylglutaryl-coa reductase inhibitors"[All Fields] OR "statin"[All Fields])) OR Atorvastatin[All Fields]) |
10 |
| ISI Web of Science, and Core Collection were searched via the web of knowledge on 1 January 2020 via apps.webofknowledge.com |
ALL FIELDS: (simvastatin) OR ALL FIELDS: (Atorvastatin) OR ALL FIELDS: (fluvastatin) OR ALL FIELDS: (statin)) AND (ALL FIELDS: (tooth movement) OR ALL FIELDS: (orthodontic)) |
12 |
| EMBASE searched via Embase on 1 January 2020 via www.embase.com |
(((orthodontic) OR tooth movement)) AND ((((simvastatin) OR fluvastatin) OR hydroximethylglutharyl coenzyme a reductase inhibitor) OR atorvastatin) |
120 |
| Scopus searched via Scopus on 1 January 2020 via https://www.scopus.com
|
TITLE-ABS-KEY (( simvastatin) OR (Atorvastatin) OR (fluvastatin) OR (statin)) AND ((tooth AND movement) OR (orthodontic)) (dent) |
99 |
| Cochrane Central Register of Controlled Trials searched via the Cochrane Library searched on 1 January 2020 via www.thecochranelibrary.com |
((simvastatin) OR (Atorvastatin) OR (fluvastatin) OR (statin)) AND ((tooth movement) OR (orthodontic)) |
3 |
| Total |
|
244 |
Eligibility Criteria
The following selection criteria were applied for this systematic review:
1. Inclusion Criteria: Randomized experimental trials and parallel and split-mouth groups were considered in this study. Articles providing data regarding the effects of statins on the OTM were considered eligible in the first analysis.
2. Exclusion Criteria: (a) in vitro histological studies, (b) review articles, case reports, and letters to editors.
Study Selection
The titles and abstracts of the searched studies were independently screened by two authors. Studies were excluded if they were either irrelevant to the current study and duplicates and/or failed to address the focused question.The controversies were resolved through discussion. Publications were included for full-text evaluations if they met the inclusion criteria in the first analysis or if insufficient information was provided in the title and abstract so that to make a decision on. A third review author was consulted where the resolution was impossible. The review authors were not blinded to the author(s), institution, or the site of the publication of all studies.
All eligible studies then underwent validity assessments and data extraction. Data were independently extracted by at least two review authors. Any disagreement was discussed and a third review author consulted where necessary. In papers that included inadequate or limited information about the OTM in the statin receivers, the corresponding authors were contacted via an e-mail for making clarification and a request for the missing data, and a reminder e-mail was sent twice after. Finally, several data were extracted from the eligible studies using extraction forms by one of the reviewers, including study design, sample size, animal spices, type and dosage of statin, method of statin administration, active tooth movement period, time of final analysis, the outcome measurement method, and the measured outcome (the amount of the OTM).
Risk of Bias in Individual Studies
The assessment of the risk of bias and the study quality of the included trials were undertaken as part of the data extraction process by at least two review authors independently and in duplicate. The risk of bias assessment was conducted using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) Tool (37). It is a two-part tool addressing ten specific domains (i.e., random sequence generation, baseline characteristics, allocation concealment, random housing, blinding of caregivers and/or investigators, random outcome assessment, blinding of assessors, incomplete outcome data, selective outcome reporting, and ‘other biases’). Each domain includes one specific entry in a ‘Risk of bias’ table. Within each entry, the first part of the tool involves reporting the events occurring in the study. The second part of the tool includes assigning a judgment relating to the risk of bias for that entry. Consequently, it was judged in terms of having a low, unclear, or high risk of bias. “Yes” and “No” judgments indicate a low and high risk of bias, respectively. The judgment will be “unclear” if insufficient details have been reported to assess the risk of bias properly. Any differences regarding the reviewers’ judgments were resolved by a third review author.
Quality of Evidence
The quality of evidence was evaluated using the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) tool (38). It addresses ten specific domains including peer-reviewed publication, control of temperature, random allocation to treatment or control, blinded induction of ischemia, blinded assessment of outcomes, and use of anesthetic without significant intrinsic neuroprotective activity. Other domains were animal model (i.e., aged, diabetic, or hypertensive), sample size calculation, compliance with animal welfare regulations, and statement of the potential conflict of interests. Each domain included one specific entry in a study quality table and assigned a “Yes” or “No” judgment for that entry. Then, each study was given a quality score out of a possible total of 10 points.
Summary Measures and Synthesis of Results
The amount of the OTM was used as the outcome measure of this meta-analysis. The mean difference (MD) with 95% confidence intervals was calculated for the continuous data (the OTM amount in millimeter). Statistical analyses were conducted using Comprehensive Meta-analysis, version 2.2. A P value less than 0.05 was considered statistically significant. The random-effect method for analysis was used to compare the outcome measure due to high heterogeneity.
The subgroup analysis for different amounts of the OTM in different methods of statin administration, different types, and dosage of statins was not performed due to the lack of sufficient similar studies.
Results
Study Selection
In total, 244 studies were found after a comprehensive search of five online databases. After removing duplicates, the titles and abstracts of the remaining 240 studies were independently screened by two authors. Two hundred and thirty-four studies were excluded based on the eligibility criteria and the PICO model in this step. Moreover, one study was a review article and thus was excluded from the investigation. The full texts of the remaining 5 studies were assessed by the same two authors.
Consequently, five studies including a total number of 108 animals were included in the qualitative assessment (39-43), and three trials were considered in the quantitative analysis (40,41,43) (Figure 1).
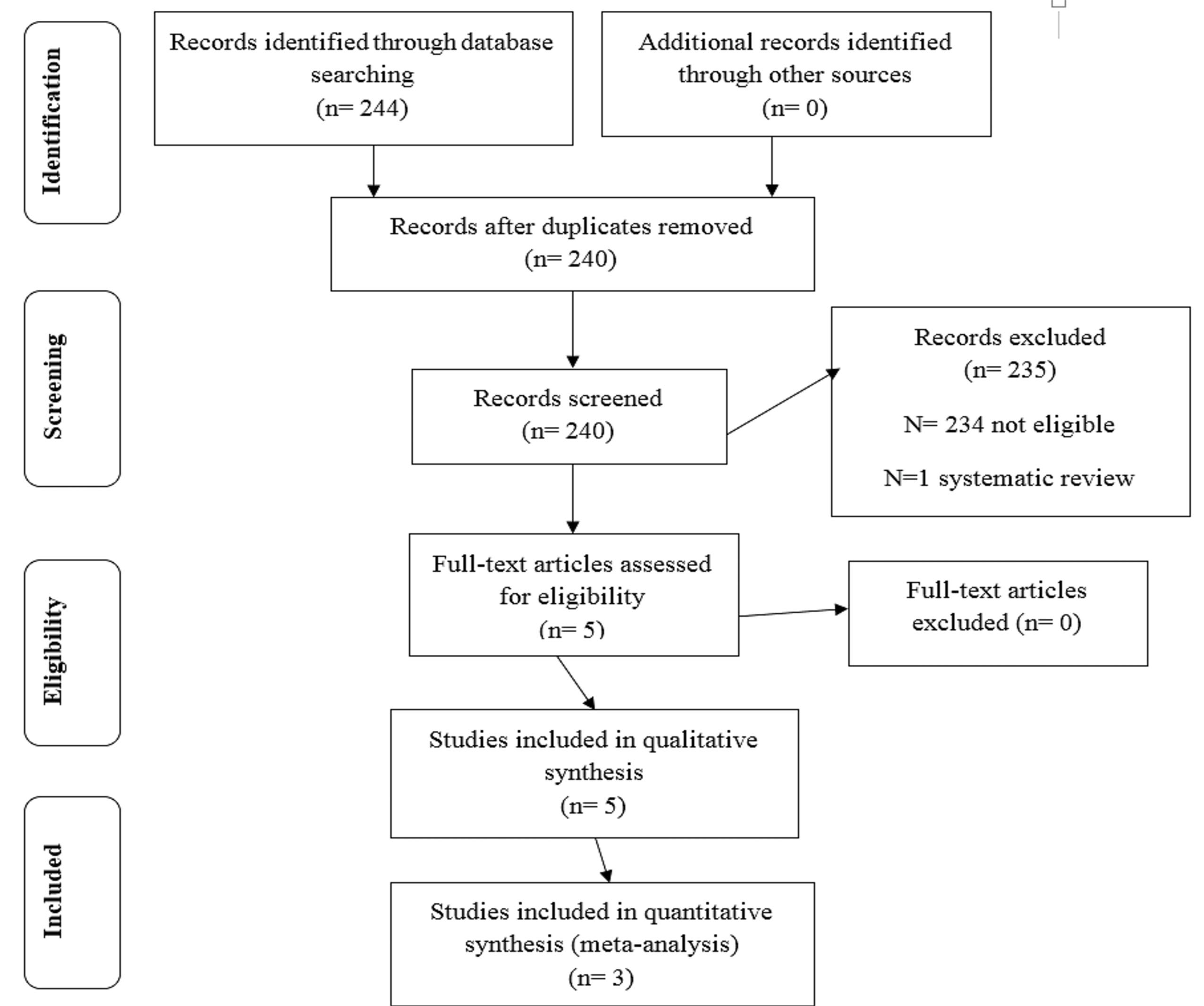
Figure 1.
PRISMA Study Flow Diagram. Note. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
.
PRISMA Study Flow Diagram. Note. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Study Characteristics
All the five included studies were randomized experimental trials (39-43), of which two cases had a split-mouth design (39,40). The other three trials used a parallel-arm design (41-43).
Study characteristics varied in most of the included studies. Variations in animal models, animal age, type and dosage of the administered statins, the method, frequency and duration of statin administration, duration and force of orthodontic treatments, and methods of outcome assessments resulted in heterogeneity among the studies.
Different subjects such as rats, dogs, and rabbits were evaluated in studies. Among the included animal studies, three trials were performed on rat models (40,41,43), one study was conducted on rabbits 39, and one study used dogs as animal models (42). The number of applied rats in trials ranged from 24 to 36. Rats aged 6 and 8-10 weeks were used in 2 studies (40,41). The mean age of the rats was not reported in one study (43). The age of rabbits was 16 weeks in one study and that of the applied dogs in another study was 10-12 months (39,42) (Table 2).
Table 2.
General Characteristics of the Included Studies
|
Author
|
Study Design
|
Study Subjects/Total Number
|
Mean Age
|
Study Groups (Number of Animals)
Case/Control
|
Primary Methods of Evaluation
|
| AlSwafeeri et al (39) |
Experimental
(split-mouth design) |
10 male New Zealand Rabbits |
16 week |
10/10 |
Impressions, casts, the 3-dimensional scanner and interproximal linear distance measurement tool (View box software), and H&E staining. |
| Dolci et al (40) |
Experimental
(split-mouth design) |
24 Male Wistar Rats |
6 week |
12/12 |
A 100 mm calibrated ruler, impressions, stone casts, and photographs;TRAP and H&E staining. |
| EsnaashariEsfahani et al (41) |
Experimental |
32 Male Wistar Rats |
8-10 weeks |
16/16 |
Histology and interproximal measurement tools |
| Mirhashemi et al (43) |
Experimental |
36 Male Sprague-Dawley Rats |
NM |
12/12 |
Histology and interproximal distance measurement tools |
| Feizbakhsh et al (42) |
Experimental |
6 Male dogs |
10-12 month |
2/2 |
A digital caliper |
Note. H&E, Hematoxylin and eosin; TRAP, tartrate-resistant acid phosphatase; NM, Not mentioned
Regarding the type of the Stains, three trials administered Simvastatin (SMV) (39,41,42) and the other two used atorvastatin (ATV) (40,43).
The studies used various modes of statin administration, including submucosal and intraligamentous injection (39), systemic (gavage) (40,43), intraperitoneal injection (41), and local injections (42).
The dosage of the applied SMV in animal model studies was 0.5 mg/0.48 mL, 0.6 mg/mL, and 0.5 mg/kg/mL (39,41,42). Two studies performed their experiments by oral administration of ATV (15 mg/kg) and 5 mg/kg ATV in carboxymethyl cellulose (CMC) (40,43). Two studies administered the statin drug locally near the teeth under investigation (39,42). Overall, the exposure duration of the subjects to statin drugs in animal studies ranged between 14 and 37 days.
The control groups in all studies underwent orthodontic treatments and were administered to non-statins such as phosphate-buffered saline, CMC, and normal saline (40-42) (Table 3).
Table 3.
Characteristics of Statin Administration in the Included Studies
|
Author
|
Case Group
|
Control Group
|
Mode of Statin Administration
|
Frequency
of Administration
|
Duration of Administration
|
| AlSwafeeri et al (39) |
Simvastatin 0/5 mg/480 µL solution
(Pluronic F127 as carrier) |
Pluronic control vehicle solution |
Two local routes:
1) 300 µL submucosal close to mesial surface of mandibular first premolar
2) 180 µL intraligamentous at mesial periodontal space of mandibular first premolar |
Weekly |
On 0, 7, and 14 days |
| Dolci et al (40) |
ATV15 mg/ kg |
0/1 mL phosphate buffered saline solution |
Gavage |
Daily |
14 days |
| Esnaashari et al (41) |
Simvastatin 2.5 mg/ kg (0.06 mg/mL solution) |
Normal saline |
Intraperitoneal injection |
Daily |
17 days |
| Mirhashemi et al43 |
ATV 5 mg/ kg in CMC |
No medication |
Gavage |
Daily |
21 days |
| Feizbakhsh et al42 |
Simvastatin 0/5 mg/kg/mL solution |
Phosphate buffered saline solution |
Local injection |
twice |
First administration: After one week of the orthodontic intervention.
Second administration: After 37 daysof the orthodontic intervention |
Note. CMC, Carboxymethyl cellulose.
Method of Orthodontic Force Application
The method of orthodontic force application was similar in three trials and they used Nickel-Titanium closed coil springs stretched between maxillary first molar and maxillary incisor (40,41,43). In one trial, animals received an orthodontic appliance consisting of nickel-titanium closed coil spring between the mandibular first premolar and mandibular incisor (39). One study used the maxillary and mandibular canines (anchorage unit) and second premolars (movement unit) to place the ligation wires (42). The applied forces ranged between 50 cN and 200 cN (Table 4).
Table 4.
Characteristics of Orthodontic Force in the Included Studies
|
Author
|
Orthodontic Appliance
|
Force
|
Site of OTM
|
Results
|
P
Value
|
| AlSwafeeri et al (39) |
13 mm Nickle-Titanium closed coil spring |
100 cN |
Between the mandibular first premolars and incisors bilaterally |
SMV administration could minimize bone resorption associated with the OTM and the number of osteoclasts. |
<0.05 |
| Dolci et al (40) |
Super elastic Nickle-Titanium closed coil spring |
50 cN |
Between the maxillary right first molar and
incisors (OTM);
Maxillary left first molar and incisors (without OTM/control) |
ATV can significantly promote osteoclast inhibition and reduce OTM in the first week.
ATV did not affect bone turnover and endochondral ossification. |
<0.05 |
| Esnaashari Esfahani et al (41) |
Nickle-Titanium closed coil spring |
50 cN |
Maxillary central incisor and maxillary first molar |
The experimental group showed a significant decrease in tooth movements, a decrease in root resorption and the number of root resorption lacunae, and the mineral appositional rate. |
<0.05 |
| Mirhashemi et al (43) |
6 mm Nickle-Titanium closed coil spring |
60 cN |
Maxillary left first molars and central incisors |
The OTM reduction following the administration of ATV was statistically significant although no significant difference was observed in the histologic variables among the three groups. |
<0.05 |
| Feizbakhsh et al (42) |
Nickle-Titanium closed coil spring |
200 cN |
Maxillary and mandibular canines
(anchorage unit) and second premolars
(movement unit) |
Although tooth movement was less in the experimental group, it was not statistically significant compared to the control group. |
>0.05 |
Note. OTM, Orthodontic tooth movement; SMV, simvastatin; ATV, Atorvastatin.
Duration of Orthodontic Tooth Movements, Time, and Method of Outcome Assessments
These parameters also varied between the included trials in this review. The duration of the OTM ranged between 7 and 21 days. The time and method of outcome assessments also varied between the trials. The amount of the OTM was reported in micrometer in one study (40), thus this amount was reported in millimeter in our analysis (Table 5).
Table 5.
Characteristics of Tooth Displacement in the Included Studies
|
Author
|
Duration of Tooth Movement
|
Teeth Measured
for Displacement
|
Magnitude of OTM in the Final Time Point Mean ± SD (mm)
|
Tooth Movement Evaluation
|
P
Value
|
|
Control Groups
|
Experimental
(Statin) Group
|
| AlSwafeeri et al (39) |
21 |
The linear distance between the first plane drawn on the distal contact area of the distal surface of the mandibular first premolar and a second plane drawn on the mesial contact area of the mesial surface of the mandibular second premolar |
1.77±0.15 |
1.04±0.38 |
Impressions recorded on days 7, 14, and 21 |
<0.05 |
| Dolci et al (40) |
21 |
Between the distal surface of the first molar and the mesial surface of the second molar at 3 points on each cast were measured on 7, 14, and 21 days |
0.48±0.06 |
0.44±0.06 |
Impressions recorded on days 7, 14, and 21 |
<0.05 |
| Esnaashari Esfahani, et al (41) |
17 |
Maxillary central incisor and maxillary first molar |
0.89±0.56 |
0.59±0.33 |
Distance between the incisor and maxillary molar was recorded using a digital caliper |
<0.05 |
| Mirhashemi et al (43) |
21 |
Measurements were performed twice (for binding purposes)
between the maxillary left first molar and the second molar |
0.58±0.22 |
0.37±0.16 |
Interproximal distance between the left upper first and second molars was measured twice by filler gauge before removing the appliance |
<0.05 |
| Feizbakhsh et al (42) |
7 |
Between canine and second premolar after one week of appliance placement and at the end of the second month |
Rate of teeth displacement per month:
Maxilla: 1.35±0.07
Mandible: 1.15±0.04 |
Rate of teeth displacement per month
Maxilla: 1.21±0.2
Mandible: 1.03±0.15 |
Digital caliper measurement on day 1 after 1 week of the orthodontic intervention and on day 60 when animals were sacrificed |
> 0.05 |
Note. OTM, Orthodontic tooth movement; SD: Standard deviation.
Risk of Bias Within Studies
A summary of the risk of bias in the included studies is presented in Figure 2. Although this study included five animal trials and 108 animals randomized to the Stains group, each of these trials had at least one domain at a high risk of bias.
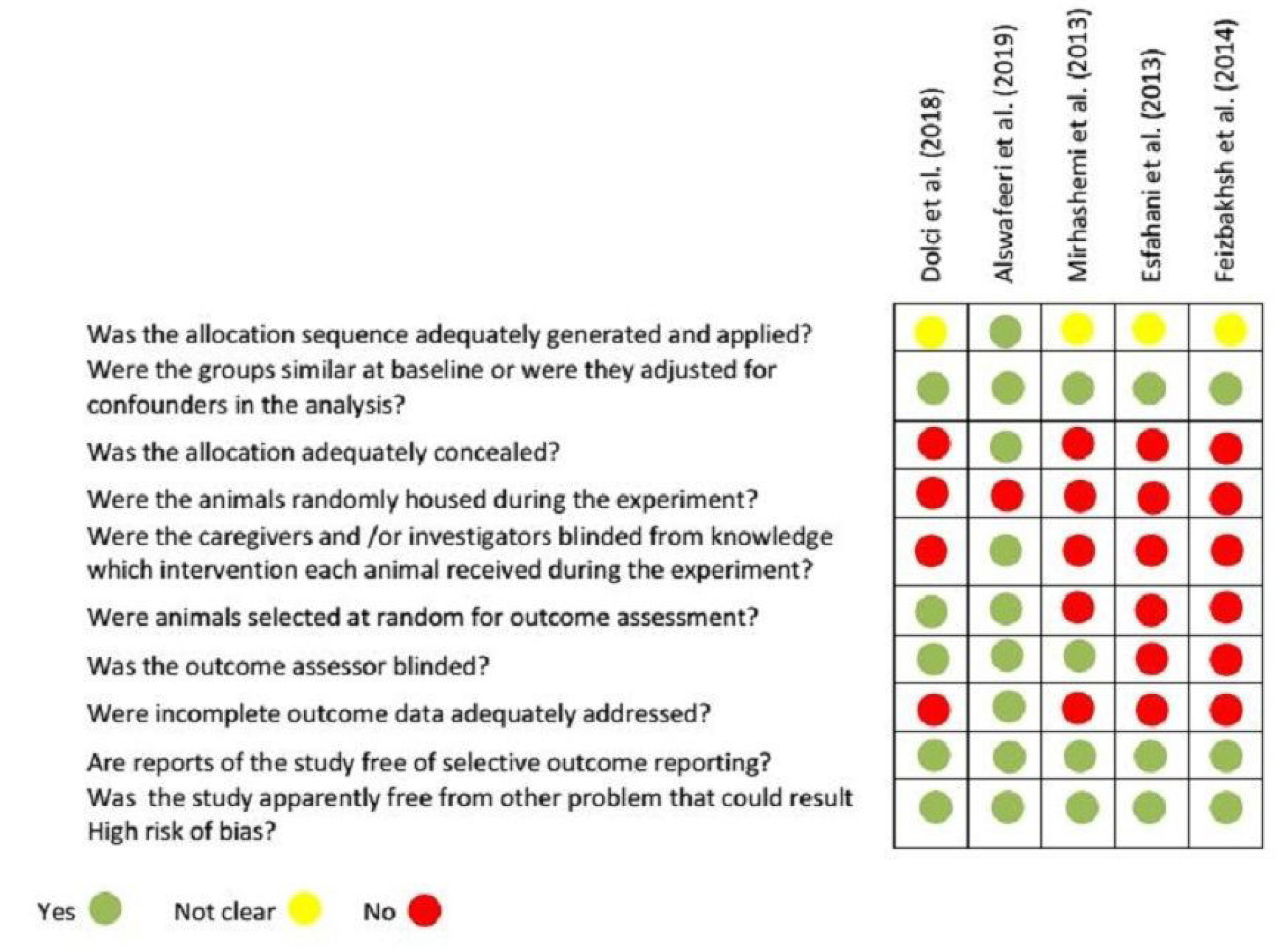
Figure 2.
Risk of Bias Summary: Authors’ Judgments About Each Risk of the Bias Item for Each Study.
.
Risk of Bias Summary: Authors’ Judgments About Each Risk of the Bias Item for Each Study.
Four of the studies did not report the method of sequence generation (selection bias) (40-43). All studies reported similar groups at baseline (selection bias) (39-43). In terms of assessing allocation concealment, only one trial clearly indicated an adequate means of allocation concealment (39). Conversely, no study described random housing (performance bias) (39-43). Only three studies announced about the blinding of caregivers, outcome assessors, or investigators (performance and detection bias) (39,40,43). Two studies reported random outcome assessment (detection bias) (39,40). Only one study adequately addressed incomplete outcome (attrition bias) (39). Finally, none of the trials had selective reporting bias.
The quality assessment of the included studies is presented in Table 6.
Table 6.
Quality Assessment of the Included Studies (CAMARADES Checklist)
|
|
Dolci et al
|
Alswafeeri et al
|
Mirhashemi et al
|
EsnaashariEsfahani et al
|
Feizbakhsh et al
|
| Publication in peer-reviewed journals |
Y |
Y |
Y |
Y |
Y |
| Statement of control of temperature |
Y |
N |
Y |
N |
N |
| Randomization of treatment or control |
Y |
Y |
Y |
Y |
Y |
| Allocation concealment |
N |
Y |
N |
N |
N |
| Blinded assessment of the outcome |
Y |
Y |
Y |
N |
N |
| Avoidance of anesthetics with marked intrinsic properties |
N |
N |
N |
N |
N |
| Use of animals with hypertension or diabetes |
N |
N |
N |
N |
N |
| Sample size calculations |
Y |
Y |
N |
N |
N |
| Statement of compliance with regulatory requirements |
Y |
Y |
Y |
N |
N |
| Statement regarding the possible conflict of interest |
N |
N |
N |
N |
N |
| Total (on 10) |
6 |
6 |
5 |
2 |
2 |
Note. CAMARADES: Collaborative approach to meta-analysis and review of animal data from experimental studies.
Results of Individual Studies and Result Synthesis
The five included studies are inconsistent in terms of their methods and the frequency of statin administration, and the type and dosage of statins. In addition, different animal models were used and different final assessment times existed between the studies. However, four studies were consistent in their methodologies regarding the measurement and comparison of the amount of the OTM between the intervention (statin receivers) and control groups (39-41,43). The heterogeneity decreased from 90.65 to 66.37 when one of these four studies was excluded from meta-analysis (39) (Figure 3), and a meta-analysis of three studies was performed for differences in the amount of OTM (40,42,43).
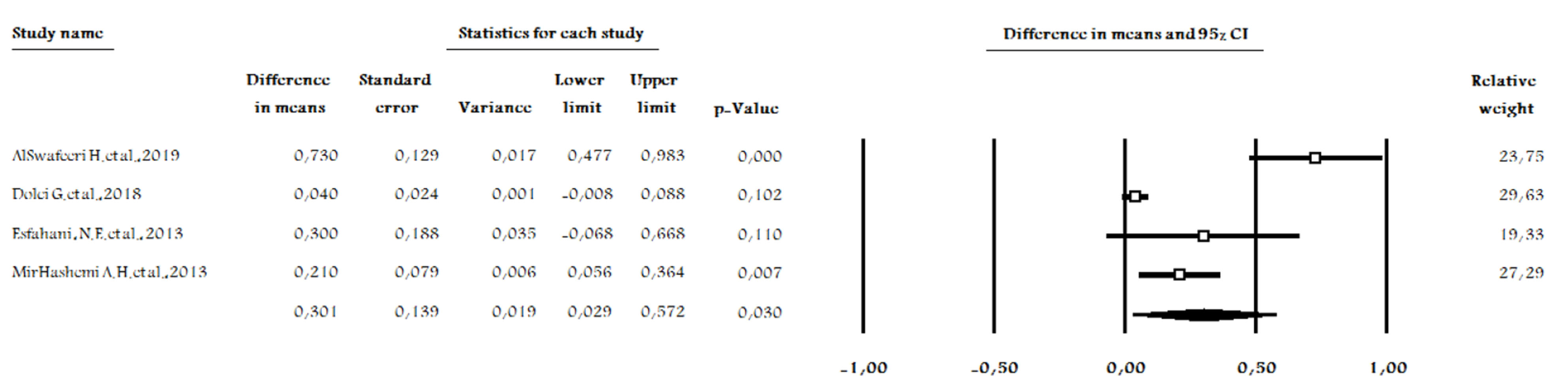
Figure 3.
Forest Plot of Four Studies, Comparing the Effects of Statins vs. Control on Orthodontic.
.
Forest Plot of Four Studies, Comparing the Effects of Statins vs. Control on Orthodontic.
Data were available comparing the outcomes at the end of the OTM. A random-effect model was performed due to differences in terms of methodologies (intervention) and the obvious heterogeneity of the data. The intervention group had a statistically insignificant lower amount of tooth movements compared to the control group (P = 0.087, MD = 0.134 (-0.020 – 0.288), degree of freedom (df) = 2, I2= 66.37, Figure 4). This results from analyzing 92 participants in three studies.
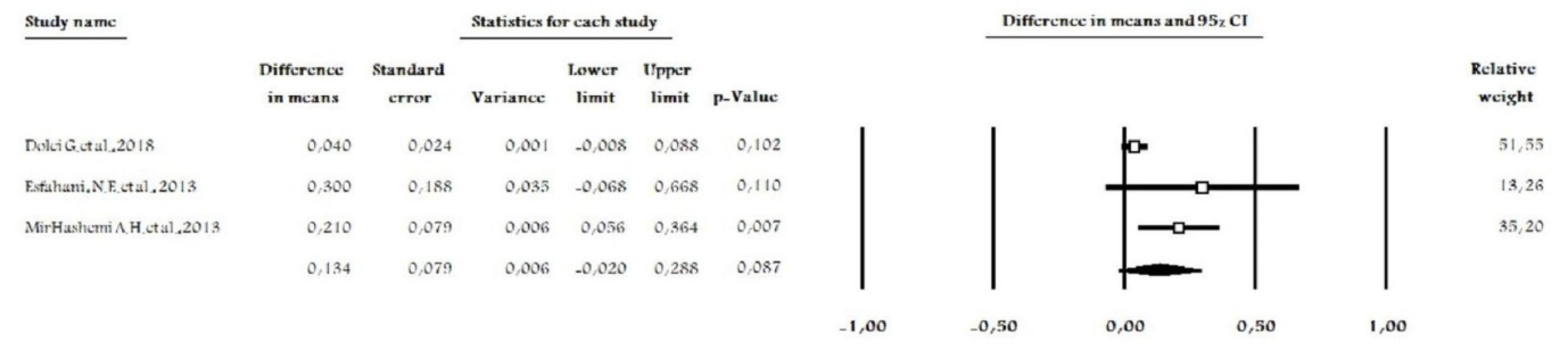
Figure 4.
Forest Plot of Three Studies, Comparing the Effects of Statins vs. Control on Orthodontic Tooth Movements.
.
Forest Plot of Three Studies, Comparing the Effects of Statins vs. Control on Orthodontic Tooth Movements.
Discussion
The aim of this study was to systematically review and evaluate whether statins decrease the OTM in animals undergoing orthodontic treatments. The literature search was comprehensive and included several databases and a hand search in the reference lists of relevant articles.
Initial evidence from four of the included studies with a high risk of bias suggested a reduction in the OTM by statin administration (39-41,43). This effect could be explained by the role of Statins in stimulating alveolar bone formation (35,44-46). The delivery of statins during OTM inhibits bone resorption through the inhibition of osteoclast cells and increases in osteoblast differentiation (19,47,48). Moreover, statins exhibit anti-inflammatory properties by inhibiting the production of certain pro-inflammatory cytokines such as IL-6 and IL-8 which are responsible for the biological reaction of periodontal tissues during OTM (49,50).
Only one study reported that OTM did not decrease following statins administration (42). This finding may be due to the frequency of statin administration, which was only twice. The number of evaluated samples in this study was also less than that of other studies. This lower sample size may result in insignificant findings.
The overall effect of statin (i.e., the type of drugs as HMGA-CoA inhibitors) on OTM magnitude was also quantitatively analyzed in this study. It should be noted that only three trials were evaluated in this meta-analysis due to heterogeneity among the included studies and their limited number, and sub-group analysis could not be undertaken in this study. The main finding of this meta-analysis is that, on average, statins do not reduce the magnitude of the OTM. This finding may be due to the limited number of included studies which results in insignificant findings.
Although the results of this study showed no difference between statin and control groups, statins reduced bone resorptive lacunas, number of osteoclasts, and generation of inflammatory interleukins such as IL-6 and IL-8 in most of our included studies and the ones related to periodontology (51,52).
Nowadays, adult patients receiving comprehensive orthodontic treatment compromise a significant number of orthodontic patients. The previously discussed properties of statins could affect OTM in adult patients who are under the treatment with statins due to their cardiovascular diseases.
In recent years, several studies have evaluated the effect of the local and systemic administration of pharmacological agents on the control of OTM (anchorage or relapse) (23-26). Thus, the local administration of the statin type of drugs could have promising effects on anchorage in orthodontic treatments if they could be evaluated in further animal and human studies.
However, reliable conclusions about statins effect on the magnitude of the OTM could not be drawn with the insufficient evidence based on the current literature, and it is important to take caution when extrapolating animal studies to humans.
Limitation of the Stud y
Although this study was performed carefully following PRISMA guidelines, several limitations remain which deserve further discussion. First, the shortage of high-quality trials is evident. Although a comprehensive literature search was performed, only three studies could be included in this analysis. Future well-designed animal and human studies are needed to obtain a more reliable conclusion. When future animal studies are planned, greater consideration should be given to study design (i.e., sequence generation, allocation concealment, random housing, blinding, and the like) in order to reduce bias.
Second, methodological heterogeneity is another limitation. Different types and dosages of statins were administered in each study. ATV and SMV, which were administered in most trials, are both lipophilic ones but have different chemical structures (53) and dosages (ranging between 2.5 mg/kg and 5 mg/kg of SMV, as well as 5 mg/kg and 15 mg/kg of ATV). The comparison of the effect of these two types of statins on OTM could not be performed due to the limited number of studies. Therefore, well-designed trials are needed to compare different types of statins and determine the most appropriate types and dosages of these drugs.
Additionally, different animal models were used in the included animal studies. Bone turnover and structure vary in different species of animals and from humans. Particularly, the bone and the root structure of rabbits are different from those of humans (54-56). In our meta-analysis, the three included trials were performed on rats. To the best of our knowledge, no human study has so far evaluated the effect of statins on OTM. Only one human study was found regarding the effect of statins on orthodontic relapse (57).
Accordingly, further evaluations of Stains should consider their potential side-effects on the turnover of other bones. Only one of the trials in this review reported both the benefits (regarding the amount of tooth movement) and the possible adverse effects (on long bone turn-over) associated with the administration of statins (40).
Conclusions
In general, the results of this meta-analysis showed that the reduction in the amount of OTM after the administration of statins was not statistically significant. Based on the information provided from the animal studies eligible for inclusion in this study, a cautionary perspective should be provided due to the low quality of evidence in this meta-analysis.
Conclusive confirmation regarding the effect of these drugs on OTM in humans is lacking since no human studies were conducted and investigations on animals cannot currently provide plausible explanations for the effects of these drugs.
Conflict of Interest Disclosures
The authors declare that they have no competing interests.
Acknowledgements
All authors have made substantive contributions to this study and all have reviewed the final paper prior to its submission.
Ethical Statement
Not applicable.
Authors’ contributions
ZA: Conceptualization, Methodology, Formal analysis, Investigation, Software, Writing- Original draft.
SHT: Conceptualization, Methodology, Formal analysis, Investigation, Writing- Review and Editing, Supervision.
FSH: Conceptualization, Formal analysis, Investigation, Writing- Review and Editing, Supervision.
All authors read and approved the final version of the paper.
Funding
This study received no contributions from private or public funding agencies.
References
- Kamińska E, Hennig M, Brandt A, Bautembach Minkowska J, Myśliwiec M. [Treatment with statins in children with familial hypercholesterolemia]. Dev Period Med 2016; 20(4):328-34. [ Google Scholar]
- Miller ML, Wright CC, Browne B. Lipid-lowering medications for children and adolescents. J Clin Lipidol 2015; 9(5 Suppl):S67-76. doi: 10.1016/j.jacl.2015.06.013 [Crossref] [ Google Scholar]
- Vuorio A, Kuoppala J, Kovanen PT, Humphries SE, Tonstad S, Wiegman A. Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev 2017; 7(7):CD006401. doi: 10.1002/14651858.CD006401.pub4 [Crossref] [ Google Scholar]
- Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci 2010; 86(5):484-93. doi: 10.2183/pjab.86.484 [Crossref] [ Google Scholar]
- Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr Jr. , Kastelein JJ, et al A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009; 360(18):1851-61. doi: 10.1056/NEJMoa0900241 [Crossref] [ Google Scholar]
- Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 2005; 4(12):977-87. doi: 10.1038/nrd1901 [Crossref] [ Google Scholar]
- Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Statins: pros and cons. Med Clin (Barc) 2018; 150(10):398-402. doi: 10.1016/j.medcli.2017.11.030 [Crossref] [ Google Scholar]
- Goes P, Lima NA, Rodrigues JA, Benevides NM, Brito GA, Lima V. Anti-inflammatory and anti-resorptive effects of atorvastatin on alveolar bone loss in Wistar rats. Braz Dent J 2016; 27(3):267-72. doi: 10.1590/0103-6440201600600 [Crossref] [ Google Scholar]
- Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999; 286(5446):1946-9. doi: 10.1126/science.286.5446.1946 [Crossref] [ Google Scholar]
- Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol 2005; 25(6):1231-6. doi: 10.1161/01.ATV.0000163840.63685.0c [Crossref] [ Google Scholar]
- Bitto A, Minutoli L, Altavilla D, Polito F, Fiumara T, Marini H. Simvastatin enhances VEGF production and ameliorates impaired wound healing in experimental diabetes. Pharmacol Res 2008; 57(2):159-69. doi: 10.1016/j.phrs.2008.01.005 [Crossref] [ Google Scholar]
- Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol 2003; 23(5):769-75. doi: 10.1161/01.atv.0000068646.76823.ae [Crossref] [ Google Scholar]
- Tahamtan S, Shirban F, Bagherniya M, Johnston TP, Sahebkar A. The effects of statins on dental and oral health: a review of preclinical and clinical studies. J Transl Med 2020; 18(1):155. doi: 10.1186/s12967-020-02326-8 [Crossref] [ Google Scholar]
- Ruan F, Zheng Q, Wang J. Mechanisms of bone anabolism regulated by statins. Biosci Rep 2012; 32(6):511-9. doi: 10.1042/bsr20110118 [Crossref] [ Google Scholar]
- Garrett IR, Mundy GR. The role of statins as potential targets for bone formation. Arthritis Res 2002; 4(4):237-40. doi: 10.1186/ar413 [Crossref] [ Google Scholar]
- Zhang Y, Bradley AD, Wang D, Reinhardt RA. Statins, bone metabolism and treatment of bone catabolic diseases. Pharmacol Res 2014; 88:53-61. doi: 10.1016/j.phrs.2013.12.009 [Crossref] [ Google Scholar]
- Liu C, Wu Z, Sun HC. The effect of simvastatin on mRNA expression of transforming growth factor-beta1, bone morphogenetic protein-2 and vascular endothelial growth factor in tooth extraction socket. Int J Oral Sci 2009; 1(2):90-8. doi: 10.4248/ijos.08011 [Crossref] [ Google Scholar]
- Liu S, Bertl K, Sun H, Liu ZH, Andrukhov O, Rausch-Fan X. Effect of simvastatin on the osteogenetic behavior of alveolar osteoblasts and periodontal ligament cells. Hum Cell 2012; 25(2):29-35. doi: 10.1007/s13577-011-0028-x [Crossref] [ Google Scholar]
- Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Effects of statins on bone mineral density: a meta-analysis of clinical studies. Bone 2007; 40(6):1581-7. doi: 10.1016/j.bone.2007.02.019 [Crossref] [ Google Scholar]
- Grasser WA, Baumann AP, Petras SF, Harwood HJ Jr, Devalaraja R, Renkiewicz R. Regulation of osteoclast differentiation by statins. J Musculoskelet Neuronal Interact 2003; 3(1):53-62. [ Google Scholar]
- Maritz FJ, Conradie MM, Hulley PA, Gopal R, Hough S. Effect of statins on bone mineral density and bone histomorphometry in rodents. Arterioscler Thromb Vasc Biol 2001; 21(10):1636-41. doi: 10.1161/hq1001.097781 [Crossref] [ Google Scholar]
- Jiang N, Guo W, Chen M, Zheng Y, Zhou J, Kim SG. Periodontal ligament and alveolar bone in health and adaptation: tooth movement. Front Oral Biol 2016; 18:1-8. doi: 10.1159/000351894 [Crossref] [ Google Scholar]
- Kaklamanos EG, Makrygiannakis MA, Athanasiou AE. Does medication administration affect the rate of orthodontic tooth movement and root resorption development in humans? a systematic review. Eur J Orthod 2020; 42(4):407-14. doi: 10.1093/ejo/cjz063 [Crossref] [ Google Scholar]
- Makrygiannakis MA, Kaklamanos EG, Athanasiou AE. Medication and orthodontic tooth movement. J Orthod 2019; 46(1 Suppl):39-44. doi: 10.1177/1465312519840037 [Crossref] [ Google Scholar]
- Zymperdikas VF, Yavropoulou MP, Kaklamanos EG, Papadopoulos MA. Effects of systematic bisphosphonate use in patients under orthodontic treatment: a systematic review. Eur J Orthod 2020; 42(1):60-71. doi: 10.1093/ejo/cjz021 [Crossref] [ Google Scholar]
- Turpin DL. Medications weigh-in on tooth movement. Am J Orthod Dentofacial Orthop 2009; 135(2):139-40. doi: 10.1016/j.ajodo.2008.12.009 [Crossref] [ Google Scholar]
- Ayukawa Y, Ogino Y, Moriyama Y, Atsuta I, Jinno Y, Kihara M. Simvastatin enhances bone formation around titanium implants in rat tibiae. J Oral Rehabil 2010; 37(2):123-30. doi: 10.1111/j.1365-2842.2009.02011.x [Crossref] [ Google Scholar]
- Ayukawa Y, Okamura A, Koyano K. Simvastatin promotes osteogenesis around titanium implants. Clin Oral Implants Res 2004; 15(3):346-50. doi: 10.1046/j.1600-0501.2003.01015.x [Crossref] [ Google Scholar]
- Du Z, Chen J, Yan F, Xiao Y. Effects of simvastatin on bone healing around titanium implants in osteoporotic rats. Clin Oral Implants Res 2009; 20(2):145-50. doi: 10.1111/j.1600-0501.2008.01630.x [Crossref] [ Google Scholar]
- Fang W, Zhao S, He F, Liu L, Yang G. Influence of simvastatin-loaded implants on osseointegration in an ovariectomized animal model. Biomed Res Int 2015; 2015:831504. doi: 10.1155/2015/831504 [Crossref] [ Google Scholar]
- Masuzaki T, Ayukawa Y, Moriyama Y, Jinno Y, Atsuta I, Ogino Y. The effect of a single remote injection of statin-impregnated poly (lactic-co-glycolic acid) microspheres on osteogenesis around titanium implants in rat tibia. Biomaterials 2010; 31(12):3327-34. doi: 10.1016/j.biomaterials.2010.01.016 [Crossref] [ Google Scholar]
- Moriyama Y, Ayukawa Y, Ogino Y, Atsuta I, Koyano K. Topical application of statin affects bone healing around implants. Clin Oral Implants Res 2008; 19(6):600-5. doi: 10.1111/j.1600-0501.2007.01508.x [Crossref] [ Google Scholar]
- Rutledge J, Schieber MD, Chamberlain JM, Byarlay M, Killeen AC, Giannini PJ. Simvastatin application to augment facial jaw bone in a dog model: pilot study. J Periodontol 2011; 82(4):597-605. doi: 10.1902/jop.2010.100214 [Crossref] [ Google Scholar]
- Seto H, Ohba H, Tokunaga K, Hama H, Horibe M, Nagata T. Topical administration of simvastatin recovers alveolar bone loss in rats. J Periodontal Res 2008; 43(3):261-7. doi: 10.1111/j.1600-0765.2007.01024.x [Crossref] [ Google Scholar]
- Wu Z, Liu C, Zang G, Sun H. The effect of simvastatin on remodelling of the alveolar bone following tooth extraction. Int J Oral Maxillofac Surg 2008; 37(2):170-6. doi: 10.1016/j.ijom.2007.06.018 [Crossref] [ Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. doi: 10.1136/bmj.b2700 [Crossref] [ Google Scholar]
- Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014; 14:43. doi: 10.1186/1471-2288-14-43 [Crossref] [ Google Scholar]
- Macleod MR, O’Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004; 35(5):1203-8. doi: 10.1161/01.str.0000125719.25853.20 [Crossref] [ Google Scholar]
- AlSwafeeri H, ElKenany W, Mowafy M, Karam S. Effect of local administration of simvastatin on orthodontic tooth movement in rabbits. Am J Orthod Dentofacial Orthop 2019; 156(1):75-86. doi: 10.1016/j.ajodo.2018.07.027 [Crossref] [ Google Scholar]
- Dolci GS, Ballarini A, Gameiro GH, Onofre de Souza D, de Melo F, Fossati ACM. Atorvastatin inhibits osteoclastogenesis and arrests tooth movement. Am J Orthod Dentofacial Orthop 2018; 153(6):872-82. doi: 10.1016/j.ajodo.2017.09.021 [Crossref] [ Google Scholar]
- Esnaashari Esfahani N, Sadeghian S, Razavi SM, Minaiyan M, Afsari E. The effects of simvastatin on bone remodeling, tooth movement and root resorption in orthodontic treatments. Biomed Pharmacol J 2013; 6(2):271-8. doi: 10.13005/bpj/414 [Crossref] [ Google Scholar]
- Feizbakhsh M, Mortazavi MS, Razavi SM, Hajhashemi V. The effects of local injection of simvastatin on tooth movement and root resorption rates under orthodontic forces in dogs. Biosci Biotechnol Res Asia 2014; 11(2):869-73. doi: 10.13005/bbra/1350 [Crossref] [ Google Scholar]
- Mirhashemi AH, Afshari M, Alaeddini M, Etemad-Moghadam S, Dehpour A, Sheikhzadeh S. Effect of atorvastatin on orthodontic tooth movement in male Wistar rats. J Dent (Tehran) 2013; 10(6):532-9. [ Google Scholar]
- Pradeep AR, Kumari M, Rao NS, Martande SS, Naik SB. Clinical efficacy of subgingivally delivered 12% atorvastatin in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2013; 84(7):871-9. doi: 10.1902/jop.2012.120393 [Crossref] [ Google Scholar]
- Pradeep AR, Rao NS, Bajaj P, Kumari M. Efficacy of subgingivally delivered simvastatin in the treatment of patients with type 2 diabetes and chronic periodontitis: a randomized double-masked controlled clinical trial. J Periodontol 2013; 84(1):24-31. doi: 10.1902/jop.2012.110721 [Crossref] [ Google Scholar]
- Stein EA, Farnier M, Waldstreicher J, Mercuri M. Effects of statins on biomarkers of bone metabolism: a randomised trial. Nutr Metab Cardiovasc Dis 2001; 11(2):84-7. [ Google Scholar]
- Yazawa H, Zimmermann B, Asami Y, Bernimoulin JP. Simvastatin promotes cell metabolism, proliferation, and osteoblastic differentiation in human periodontal ligament cells. J Periodontol 2005; 76(2):295-302. doi: 10.1902/jop.2005.76.2.295 [Crossref] [ Google Scholar]
- Maeda T, Matsunuma A, Kurahashi I, Yanagawa T, Yoshida H, Horiuchi N. Induction of osteoblast differentiation indices by statins in MC3T3-E1 cells. J Cell Biochem 2004; 92(3):458-71. doi: 10.1002/jcb.20074 [Crossref] [ Google Scholar]
- Nogueira AV, de Molon RS, Nokhbehsaim M, Deschner J, Cirelli JA. Contribution of biomechanical forces to inflammation-induced bone resorption. J Clin Periodontol 2017; 44(1):31-41. doi: 10.1111/jcpe.12636 [Crossref] [ Google Scholar]
- Kommuri K, Javed F, Akram Z, Khan J. Effect of statins on orthodontic tooth movement: a systematic review of animal and clinical studies. Arch Oral Biol 2020; 111:104665. doi: 10.1016/j.archoralbio.2020.104665 [Crossref] [ Google Scholar]
- Akram Z, Vohra F, Javed F. Efficacy of statin delivery as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a meta-analysis. J Investig Clin Dent 2018; 9(2):e12304. doi: 10.1111/jicd.12304 [Crossref] [ Google Scholar]
- Bertl K, Steiner I, Pandis N, Buhlin K, Klinge B, Stavropoulos A. Statins in nonsurgical and surgical periodontal therapy A systematic review and meta-analysis of preclinical in vivo trials. J Periodontal Res 2018; 53(3):267-87. doi: 10.1111/jre.12514 [Crossref] [ Google Scholar]
- Gazzerro P, Proto MC, Gangemi G, Malfitano AM, Ciaglia E, Pisanti S. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev 2012; 64(1):102-46. doi: 10.1124/pr.111.004994 [Crossref] [ Google Scholar]
- Ibrahim AY, Gudhimella S, Pandruvada SN, Huja SS. Resolving differences between animal models for expedited orthodontic tooth movement. Orthod Craniofac Res 2017; 20 Suppl 1:72-6. doi: 10.1111/ocr.12175 [Crossref] [ Google Scholar]
- Ren Y, Maltha JC, Kuijpers-Jagtman AM. The rat as a model for orthodontic tooth movement--a critical review and a proposed solution. Eur J Orthod 2004; 26(5):483-90. doi: 10.1093/ejo/26.5.483 [Crossref] [ Google Scholar]
- Castañeda S, Largo R, Calvo E, Rodríguez-Salvanés F, Marcos ME, Díaz-Curiel M. Bone mineral measurements of subchondral and trabecular bone in healthy and osteoporotic rabbits. Skeletal Radiol 2006; 35(1):34-41. doi: 10.1007/s00256-005-0022-z [Crossref] [ Google Scholar]
- Jahanbin A, Abtahi M, Namdar P, Heravi F, Sadeghi F, Arab H. Evaluation of the effects of subgingival injection of Simvastatin on space re-opening after orthodontic space closure in adults. J Dent Res Dent Clin Dent Prospects 2016; 10(1):3-7. doi: 10.15171/joddd.2016.001 [Crossref] [ Google Scholar]