Avicenna J Dent Res. 12(4):107-111.
doi: 10.34172/ajdr.2020.22
Original Article
An In Vitro Analysis of the Antifungal Effect of Nystatin and Fluconazole Incorporated into Tissue Conditioner with and Without Using Varnish Containing Self-cured Resin and 1,1,1-Trichloroethane
Shima Ghasemi 1  , Safa Raeesi 2
, Safa Raeesi 2  , Katayoun Sadr 1
, Katayoun Sadr 1  , Azra Kiafar 3
, Azra Kiafar 3  , Amir Reza Babaloo 4, *
, Amir Reza Babaloo 4, * 
Author information:
1Assistant Professor, Department of Prosthodontics, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran.
2Dental Student, Faculty of Dentistry, Tabriz University of Medical Science, Tabriz, Iran.
3Dental Student, Faculty of Dentistry, Tabriz University of Medical Science Tabriz, Iran.
4Assistant Professor, Department of Periodontics, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran.
Abstract
Background: Incorporating antifungal drugs into liners has been proposed to treat denture stomatitis. Varnish application on tissue conditioners can decrease the porosities and irregularities, biofilm, and pathogens adhesion. In this study, we evaluated the effect of varnish application on releasing the antifungal drugs incorporated into tissue conditioners.
Methods: Pure form of nystatin and fluconazole were mixed into tissue conditioner powder separately at 5% wt/wt concentration and prepared according to manufacturer’s instruction. Then, disk-shaped specimens (5 mm in diameter and 1 mm in thickness) were prepared at 30 nystatin and 30 fluconazole specimens. Varnish (containing 50 mL of 1,1,1-trichloroethane and 3 ml of self-cured resin) was applied on the surface of 15 disks of each drug and the other specimens were used as the control group (without varnish). Next, the disks were put in agar plates cultured with standard Candida albicans and incubated for 7 days. Mean inhibition diameter for each disk was measured with digital caliper at 24 hours, 3 days, and 7 days. Each step was performed in triplicate. Data was analyzed with one-way ANOVA and Friedman, Wilcoxon, and Mann-Whitney U tests.
Results: The mean inhibition diameter (MID) at days 1, 3, and 7 in fluconazole without varnish group was 12.63, 3.90, and 3.67, respectively; in fluconazole with varnish was 3.00, 2.50, and 2.50, respectively; in nystatin without varnish was 5.78, 3.90, and 3.87, respectively; in nystatin with varnish group was 2.50, 0.00, and 0.00, respectively. fluconazole without varnish group exhibited significantly higher MID and nystatin with varnish group had lower MID.
Conclusions: In this experimental study, fluconazole was more effective than nystatin. In groups without varnish, antifungal effect continued up to day 7. Using varnish in tissue conditioner can decrease antifungal effect.
Keywords: Nystatin, Fluconazole, Varnish, Antifungal
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Ghasemi S, Raeesi S, Sadr K, Kiafar A, Babaloo AR. An in vitro analysis of the antifungal effect of nystatin and fluconazole incorporated into tissue conditioner with and without using varnish containing self-cured resin and 1,1,1-trichloroethane. Avicenna J Dent Res. 2020;12(4):107-111. doi: 10.34172/ajdr.2020.22.
Background
Highlights
-
Examination of the antifungal effect of nystatin from tissue conditioner in the group without varnish showed that the mean inhibition diameter (MID) at days 1, 3, and 7 was 5.86 mm, 3.9 mm and 3.86 mm, respectively.
-
At day 3, there was a significant decrease in drug release. However, at day 7, the drug release did not decrease significantly compared to day 3.
-
The highest antifungal effect of nystatin was observed in the group without varnish at day 1.
The use of removable denture can cause many changes in the oral cavity. Chronic atrophic candidiasis is considered as one of the most common complications of removable denture, which is also known as denture stomatitis or denture sore mouth. (1) The fungal growth on the denture surface is one of the main causes of burning mouth. Poor oral hygiene and continuous use of dentures are predisposing factors of fungal growth. Moreover, the lack of antifungal activity of denture and the high surface roughness are among factors which accelerate fungal growth. Candida albicans adherence on denture base can cause denture stomatitis, especially when it is continuously used by people with trauma and poor oral hygiene. Hygiene control, which leads to better denture adaptation, and regeneration of denture-bearing tissues are among the measures used to prevent and treat denture stomatitis. (2) In order to treat fungal infection, drug treatment is required along with cleaning the complete denture through brushing. On the other hand, restrictions on topical administration of drugs include being washed away by saliva and reduced concentration at the site of operation. Systemic administration also requires high doses along with serious side effects (3).
Denture soft liners are fitted between surfaces of a removable denture. These materials have a time-dependent cushioning effect, and improve healing traumatized tissues and retention of intraoral and extraoral removable dentures. These materials are generally divided into two categories: short-term soft liners (tissue conditioners) and long-term soft liners. The soft and flexible nature of these substances makes them an appropriate alternative for diagnostic, adjuvant, and therapeutic purposes in the treatment of removable denture patients. Ideally, to ensure adequate cushioning property, a tissue conditioner must be replaced every 3-4 days, and this process should continue until the tissues are fully healed. In case of no patient referral, non-replaced tissue conditioners will cause many problems, including pain reported by the patient, recurrent tissue irritation, bad breath, isolated liners, and base denture contaminated with adhering microorganisms (4).
As time passes and the physical properties of a tissue conditioner change, its surface integrity and viscoelasticity are affected and the increased biofilm accumulation is seen; as a result, the material loses its effectiveness (5).
Many solutions have been proposed to prevent biofilm accumulation and growth of fungi, including Candida on tissue conditioners. They include incorporation of various antifungal drugs into the tissue conditioner (3,6), application of varnish containing antifungal drug (7,8),or immersion of a denture relined with a tissue conditioner in disinfectant solutions (9).
Using disinfectant or drug solutions is not always beneficial and can have adverse effects on the physical properties of soft liners. (10,11) Furthermore, the use of regular home medication depends on the patients, and using disinfectant solution may have unpredictable clinical results. There are numerous studies about incorporation of antifungal drugs or disinfectants into soft liners. (2,3,6) The results of some studies show that the addition of medications to soft liners is beneficial for antifungal activity if adequate attention is paid to precise proportions, and it has no effect on physical properties. (3,12) It is believed that some varnishes used on tissue conditioners improve their physical properties and increase their lifetime and efficiency (4).
This study was conducted to investigate the effect of varnish application on releasing antifungal drug incorporated into tissue conditioners.
Materials and Methods
This research was performed as an experimental laboratory study. The present study included samples of GC tissue conditioner (GC corporation Tokyo, Japan) containing the antifungal drugs nystatin (Sigma-Aldrich, St. Louis, USA) and fluconazole (Sciencelab, 14025 Smith Rd, Houston, Texas).
Cylindrical molds with 5 mm diameter and 1 mm thickness were prepared according to the manufacturer’s instructions.
After preparing samples, varnish containing two ingredients, trichloroethane (Sigma-Aldrich, St. Louis, USA) and self-curing acrylic resin (Triplex cold –Ivoclar Vivadent AG, FL-9494 Schaan, Liechtenstein), was applied and antifungal effect of samples were evaluated.
Sample Size and Sampling Method
The study results of Sharma and Hegde were used to determine the sample size (13). Considering α = 0.05, the power was 80%, and 8 differences were observed in the MID of 15 samples in each group.
Description of the Study Groups
Control group: Fifteen discs of tissue conditioner combined with nystatin and 15 discs of tissue conditioner combined with fluconazole without varnish containing self-hardening acrylic resin and 1,1,1-trichloroethane solution on the surface.
Test group: Fifteen discs of tissue conditioner combined with nystatin and 15 discs of tissue conditioner combined with fluconazole with varnish containing self-hardening acrylic resin and 1,1,1-trichloroethane solution on the surface.
In order to prepare varnish, 50 mL of 1,1,1-trichloroethane solution with 2 mL of self-curing acrylic resin powder were poured in a glass-lidded bottle. It took several hours for the powder to be fully dissolved in the solvent. Finally, the resulting material was viscose like glycerin.
Candida albicans standard strain (ATCC 10231) (Pasteur Institute, Iran) was immersed in Sabouraud Dextrose Agar (SDA) medium and cultured at 37°C. After 8 hours, the C. albicans suspension was diluted by sterile broth to reach a density equal to standard barium sulfate according to 0.5 McFarland tube number standards. The suspension was also placed on the slide and the colony-forming unit (CFU) of fungi was measured at 1.5 × 1018 using a microscope (13).
Diluted C. albicans solution (0.5 mL) was poured into sterile SDA plates and lawn culture was applied. In this method, whole plate surface is covered by a fungal suspension and a uniform surface is provided for fungal growth. This technique is effective for doing antibiotic sensitivity tests (14).
The tissue conditioners were weighed according to the manufacturer’s instructions and fluconazole for fabrication of specimens (5% wt/wt) and nystatin (5% wt/wt) (Sigma-Aldrich, St. Louis, USA) was separately added to the polymeric phase (powder) of the material. Then, 0.12 g of each drug and 2.28 g of tissue conditioner powder using a sensitive scale were measured and mixed with the liquid for 30 seconds. After mixing the material using a 1 mm thick acrylic stopper, the disc-shaped specimens (5 mm diameter and 1 mm thickness) were fabricated by a cylindrical generator between two glass slabs (2).
Therefore, a total of 60 discs were prepared from the tissue conditioner (GC, Tokyo, Japan) and fluconazole (5% wt/wt) and nystatin (5% wt/wt) were incorporated in all discs. Then, varnishes were applied in 15 specimens of each drug group using brushes and 15 specimens without varnish were considered as the control group (13). The discs were placed in the culture medium (3 plates per disc) and the plates were incubated at 37°C for 7 days. The MID of each disc was measured in mm from the middle of the disc by a metal ruler after 24 hours, 3 days, and 7 days. All stages and experiments were conducted in triplicate for closer examination.
Statistical Analysis
The Friedman test was used for a pairwise comparison on the antifungal properties of nystatin and fluconazole, which are incorporated into tissue conditioners in varnish and non-varnish groups. Wilcoxon test was used to compare the antifungal properties of the drugs during the study days. Mann-Whitney test was used to compare the antifungal effect of fluconazole and nystatin (separately) in varnish and non-varnish groups. Statistical analysis was performed using SPSS statistical software version 17 (SPSS Inc., Chicago, Ill., USA). The P value < 0.05 was considered as statistically significant.
Results
Evaluating the antifungal effect of nystatin, which was incorporated into tissue conditioners in non-varnish group, showed that MID was equal to 5.86, 3.9, and 3.86 mm at days 1, 3, and 7, respectively. There was a significant difference in antifungal activity at days 1, 3, and 7 based on Friedman test (P = 0.001).
A pairwise comparison of antifungal properties on the studied days (Wilcoxon test) showed a significant decrease in MID at day 3 (P = 0.001). However, the drug release rate was not significantly different at days 7 and 3.
In other words, the highest antifungal activity of nystatin, which was incorporated into tissue conditioner in the varnish group was seen at day 1.
Evaluating the antifungal properties of nystatin incorporated into the tissue, which was conditioners in the varnish group, showed that MID was equal to 2.5 mm at day 1 (equal to the disk size). Releasing the drug was stopped and the fungus covered the disc surface at days 3 and 7. According to the results of Friedman test, there was a significant difference between the two groups in terms of their antifungal properties at days 1, 3, and 7 (P = 0.001).
A pairwise comparison of the antifungal properties on the studied days (Wilcoxon test) indicated that the antifungal effect of the drug was stopped at day 3.
In other words, the highest antifungal activity of nystatin, which was incorporated into the tissue conditioners in the varnish group, was seen at day 1.
Evaluating the antifungal properties of fluconazole, which was incorporated into the tissue conditioners in the varnish group, showed that the mean drug release was 3 mm at day 1, 2.5 mm at day 3 (equal to disc size), and 2.5 mm at day 7 (equal to disk size). Friedman test showed a significant difference in antifungal activity at days 1, 3, and 7 (P = 0.001).
Pairwise comparison of the antifungal properties on the studied days (Wilcoxon test) showed a significant reduction in drug release at day 3 (P = 0.002). Nevertheless, the antifungal effect of the drug did not change significantly at day 7, compared to day 3.
In other words, the highest antifungal properties of fluconazole, which was incorporated into the tissue conditioners in the varnish group, were measured at day 1.
Evaluating antifungal properties of fluconazole, which was incorporated into the issue conditioners in non-varnish group, showed that the mean antifungal drug release was 12.63, 3.9, and 3.66 mm at days 1, 3, and 7, respectively. The results of Friedman test showed a significant difference in antifungal properties of this drug at days 1, 3, and 7 (P = 001).
Pairwise comparison of antifungal properties on the studied days (Wilcoxon test) showed a significant reduction in drug release at day 3 (P = 0.001); however, there was no significant difference in antifungal effect of this drug at days 7 and 3.
In other words, the highest antifungal effect of fluconazole, which was incorporated into the tissue conditioners in the non-varnish group, was seen at day 1 (Table 1 and Figure 1).
Table 1.
Comparison of the Antifungal Effect of 4 Different Substrates in 3 Different Days (day 1, day 3, and day 7)
|
|
Day 1
|
Day 3
|
Day 7
|
|
Mean
|
SD
|
Mean
|
SD
|
Mean
|
SD
|
| Fluconazole without varnish |
12.63 |
0.92 |
3.90 |
0.60 |
3.67 |
0.75 |
| Fluconazole with varnish |
3.00 |
0.38 |
2.50 |
0.00 |
2.50 |
0.00 |
| Nystatin without varnish |
5.87 |
0.92 |
3.90 |
0.76 |
3.87 |
0.67 |
| Nystatin with varnish |
2.50 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
|
P value |
<0.001 |
<0.001 |
<0.001 |
P value obtained from one-way ANOVA test.
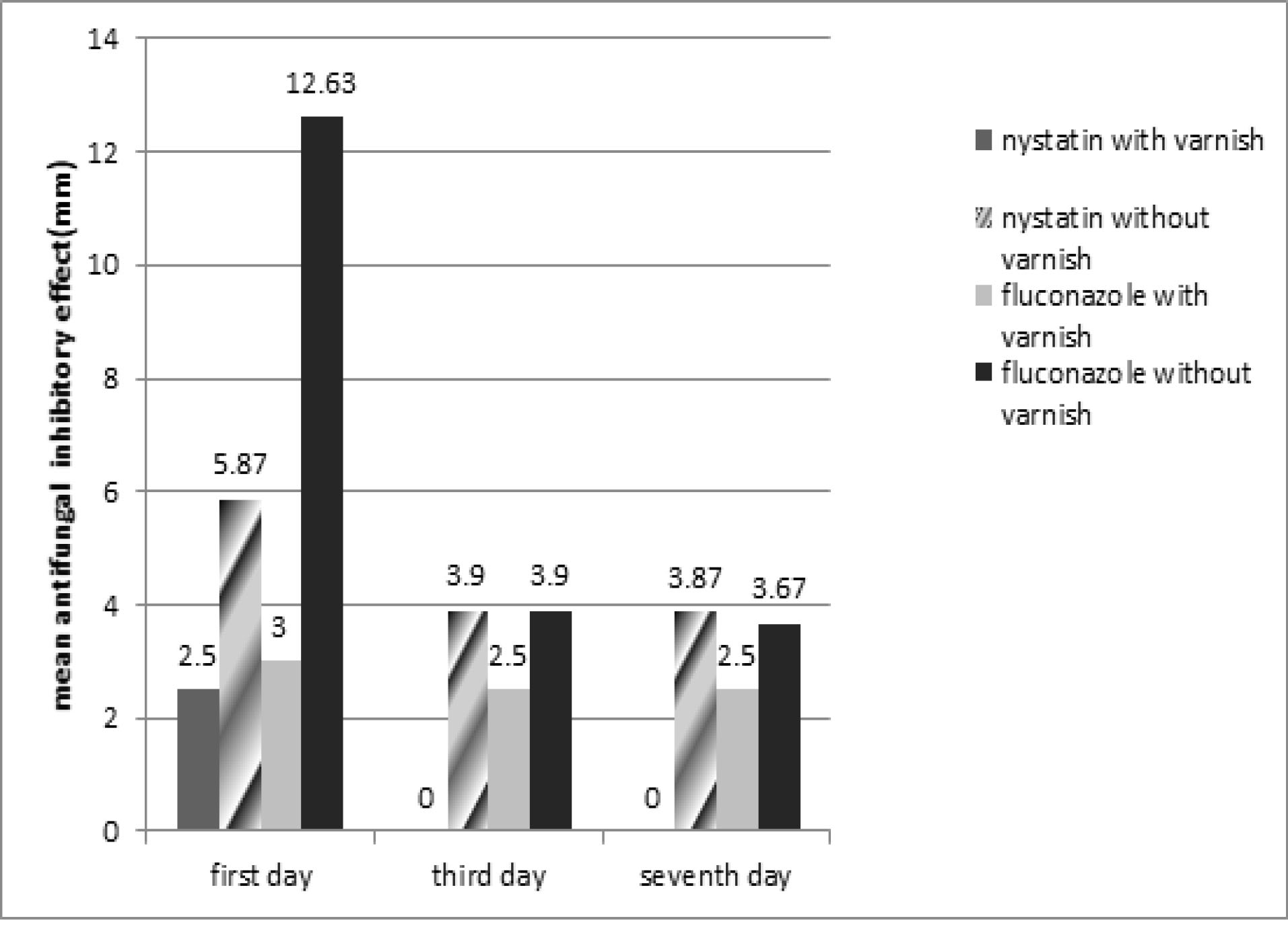
Figure 1.
Comparison the Antifungal Effect in 4 Groups in Day1, Day 3, Day 7.
.
Comparison the Antifungal Effect in 4 Groups in Day1, Day 3, Day 7.
Discussion
Removable dentures can cause many changes in the mouth. Chronic atrophic candidiasis is considered as one of the common complications of using removable prostheses, which is known as denture stomatitis or denture sore mouth prevalent in up to 15% of denture wearers (15,16).
Active pathogens and microorganisms begin to colonize and proliferate in denture base and the space between the mucosa and the denture surface due to prolonged use of the prosthesis.
The etiology of denture stomatitis is multifactorial in nature. Predisposing factors include denture-related trauma, poor denture hygiene, dietary factors, dry mouth, continuous use of denture without removing it, acute diseases, and problems of immune system. However, C. albicans is known as the first pathogenic microorganism which is normally isolated from patients who suffer from denture stomatitis. C. albicans is one of the normal oral microflora components. Therefore, it is normally seen in the oral cavity; but it can be pathogenic in some people with immunodeficiency or chronic local irritation (17).
Denture stomatitis treatment should include the following: replacement of old dentures, elimination of anatomical abnormalities, atraumatic occlusion, nutritional restitution, oral hygiene education, systematic evaluation, and antifungal therapy. Mechanical plaque control and proper denture use are among important criteria in the prevention and treatment of disease (17).
Tissue conditioners or temporary soft liners are used to increase denture adaptation and allow repair tissues near the denture base. The elasticity properties of tissue conditioners can lead to flexible fusion forces, and thus help to allow tissue repair (17). Previous studies showed that these materials should be replaced every 3 to 4 days to ensure maximum clinical efficacy of antifungal drugs incorporated into tissue conditioners (4). Antifungal therapy must be started, if fungal organisms are identified.
Triazole antifungals (fluconazole and itraconazole) play an important role in the treatment of denture stomatitis. Since most patients do not properly use topical antifungal drugs in the oral cavity, these drugs are incorporated into tissue conditioners. Various studies have shown the significant antifungal effect of this medication on the C. albicans growth. (17)
Application of varnishes on the surface of tissue conditioners can also reduce porosity and roughness of the denture surface and increase the lifetime of these materials. Not also these factors reduce porosity and roughness, but also they reduce biofilm accumulation and pathogen formation (18).
Since fluconazole and nystatin are the most commonly used antifungal drugs in the treatment of oral candidiasis (2,6), in the present study, they were incorporated in the tissue conditioners.
Falah-Tafti et al showed that the potential antifungal activity of nystatin, when incorporated into tissue conditioners, was greater than fluconazole (2).
Most tissue conditioners have the highest effect after 24 to 72 hours. Graham reported that tissue conditioners have flow properties up to 7 days (17).
Most studies have used different weight percentages of antifungal drugs, which were incorporated into tissue conditioners alone and without varnish. The most effective weight percentage (w/t%) varies depending on the drug type (1,2,12,19,20). In some studies, the antifungal drugs and tissue conditioners were used and relevant evaluations were made at different intervals (3,6,17). The antifungal drugs and varnish have been used in other studies.
Kumar et al found that nystatin was more effective than fluconazole as an antifungal drug against C. albicans in patients with denture stomatitis (21).
In a study by Barua et al leaf extract in combination with tissue conditioner showed significant potential as an effective antimicrobial agent against C. albicans and Streptococcus mutans (22).
According to Krishnamoorthy et al, the composition of 10% w/w coconut oil and viscogel tissue conditioner showed a significant reduction in C. albicans growth (23).
This study attempted to investigate the effect of varnish containing self-curing acrylic resin and 1,1,1-trichloroethane solution on releasing antifungal drugs incorporated into tissue conditioners in complete dentures. The results of the experiment showed the greatest antifungal effect of nystatin and fluconazole in the varnish and non-varnish groups at day 1. In addition, at day 3, the drug release was stopped and conditions remained the same until day 7. Pairwise comparison of the antifungal effect of fluconazole and nystatin in the varnish-treated and untreated groups (Mann-Whitney test) showed that the antifungal effect of the drugs in the varnish-treated groups was significantly greater than in the untreated groups at days 1, 3, and 7.
Conclusions
The main findings of the current study include:
-
The denture should be replaced every 3 to 4 days in order to ensure maximum antifungal efficacy of the antifungal drug incorporated into the tissue conditioner. However, antifungal activity in the untreated groups lasts up to 7 days.
-
The effect of antifungal drug may be reduced during using both varnish and tissue conditioner. Considering the clinical purpose of using a tissue conditioner in each patient, we should evaluate the advantages and disadvantages of varnish and then make a decision.
-
The present in vitro study indicated that fluconazole is more effective than nystatin in terms of antifungal activity.
Conflict of Interest Disclosures
The authors declare no conflict of interests.
Acknowledgments
The authors would like to acknowledge the Dental and Periodontal Research Center at Tabriz University of Medical Sciences, Iran for financial support.
Ethical Statement
The Research Council of the Faculty of Dentistry, Tabriz University of Medical Sciences, Iran approved the present study (code: IR.TBZMED.VCR.REC.1394.865).
Authors’ Contribution
SG, SR, KS, AK, AB and SG designed and performed experiments, analyzed data and co-wrote the paper. SR, KS and AK performed the experiments. AK and KS performed statistical analyses.SG, SR and AB supervised the research. AB and SR designed experiments and co-wrote the paper. SG, SR and AB drafted and provided critical revision of the article.
References
- Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis Aetiology and management: a review Part 1 Factors influencing distribution of Candida species in the oral cavity. Aust Dent J 1998; 43(1):45-50. doi: 10.1111/j.1834-7819.1998.tb00152.x [Crossref] [ Google Scholar]
- Falah-Tafti A, Jafari AA, Lotfi-Kamran MH, Fallahzadeh H, Hayan RS. A comparison of the efficacy of nystatin and fluconazole incorporated into tissue conditioner on the in vitro attachment and colonization of Candida albicans. Dent Res J (Isfahan) 2010; 7(1):18-22. [ Google Scholar]
- Srivatstava A, Ginjupalli K, Perampalli NU, Bhat N, Ballal M. Evaluation of the properties of a tissue conditioner containing origanum oil as an antifungal additive. J Prosthet Dent 2013; 110(4):313-9. doi: 10.1016/s0022-3913(13)60381-9 [Crossref] [ Google Scholar]
- Zarb GA, Hobkirk JA, Eckert SE, Jacob RF. Prosthodontic Treatment for Edentulous Patients: Complete Dentures and Implant-Supported Prostheses. 13th ed. St. Louis: Mosby; 2013. p. 156,163,392,394.
- Ebadian B, Navarchian AH, Sedighipour L. The effect of surface coating on softness of two kinds of tissue conditioners. Dent Res J 2006; 3(1):1-6. [ Google Scholar]
- Chow CK, Matear DW, Lawrence HP. Efficacy of antifungal agents in tissue conditioners in treating candidiasis. Gerodontology 1999; 16(2):110-8. doi: 10.1111/j.1741-2358.1999.00110.x [Crossref] [ Google Scholar]
- Milillo L, Lo Muzio L, Carlino P, Serpico R, Coccia E, Scully C. Candida-related denture stomatitis: a pilot study of the efficacy of an amorolfine antifungal varnish. Int J Prosthodont 2005; 18(1):55-9. [ Google Scholar]
- Czerninski R, Pikovsky A, Gati I, Friedman M, Steinberg D. Comparison of the efficacy of a novel sustained release clotrimazole varnish and clotrimazole troches for the treatment of oral candidiasis. Clin Oral Investig 2015; 19(2):467-73. doi: 10.1007/s00784-014-1259-5 [Crossref] [ Google Scholar]
- Huh JB, Lim Y, Youn HI, Chang BM, Lee JY, Shin SW. Effect of denture cleansers on Candida albicans biofilm formation over resilient liners. J Adv Prosthodont 2014; 6(2):109-14. doi: 10.4047/jap.2014.6.2.109 [Crossref] [ Google Scholar]
- Tan H, Woo A, Kim S, Lamoureux M, Grace M. Effect of denture cleansers, surface finish, and temperature on Molloplast B resilient liner color, hardness, and texture. J Prosthodont 2000; 9(3):148-55. [ Google Scholar]
- Farzin M, Bahrani F, Adelpour E. Comparison of the effect of two denture cleansers on tensile bond strength of a denture liner. J Dent (Shiraz) 2013; 14(3):130-5. [ Google Scholar]
- Bertolini MM, Portela MB, Curvelo JA, Soares RM, Lourenço EJ, Telles DM. Resins-based denture soft lining materials modified by chlorhexidine salt incorporation: an in vitro analysis of antifungal activity, drug release and hardness. Dent Mater 2014; 30(8):793-8. doi: 10.1016/j.dental.2014.05.004 [Crossref] [ Google Scholar]
- Sharma S, Hegde V. Comparative evaluation of antifungal activity of melaleuca oil and fluconazole when incorporated in tissue conditioner: an in vitro study. J Prosthodont 2014; 23(5):367-73. doi: 10.1111/jopr.12117 [Crossref] [ Google Scholar]
- Gupte S. The Short Textbook of Medical Microbiology for Dental Students. 1st ed. Jaypee Brothers Medical Publishers; 2012. p. 36.
- Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis Aetiology and management: a review Part 2 Oral diseases caused by Candida species. Aust Dent J 1998; 43(3):160-6. doi: 10.1111/j.1834-7819.1998.tb00157.x [Crossref] [ Google Scholar]
- Budtz-Jörgensen E, Stenderup A, Grabowski M. An epidemiologic study of yeasts in elderly denture wearers. Community Dent Oral Epidemiol 1975; 3(3):115-9. doi: 10.1111/j.1600-0528.1975.tb00291.x [Crossref] [ Google Scholar]
- Monzavi A, Siadat H, Atai M, Alikhasi M, Nazari V, Sheikhzadeh S. Comparative evaluation of physical properties of four tissue conditioners relined to modeling plastic material. J Dent (Tehran) 2013; 10(6):506-15. [ Google Scholar]
- Mainieri VC, Beck J, Oshima HM, Hirakata LM, Shinkai RS. Surface changes in denture soft liners with and without sealer coating following abrasion with mechanical brushing. Gerodontology 2011; 28(2):146-51. doi: 10.1111/j.1741-2358.2010.00375.x [Crossref] [ Google Scholar]
- Martínez A, Rojas N, García L, González F, Domínguez M, Catalán A. In vitro activity of terpenes against Candida albicans and ultrastructural alterations. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 118(5):553-9. doi: 10.1016/j.oooo.2014.07.009 [Crossref] [ Google Scholar]
- Geerts GA, Stuhlinger ME, Basson NJ. Effect of an antifungal denture liner on the saliva yeast count in patients with denture stomatitis: a pilot study. J Oral Rehabil 2008; 35(9):664-9. doi: 10.1111/j.1365-2842.2007.01805.x [Crossref] [ Google Scholar]
- Kumar N, Kumari A, Priyadarshi V, Kumar A, Prasad RS, Kumar B. A comparative efficacy of nystatin and fluconazole incorporated into tissue conditioner as drug delivery method for Denture stomatitis. J Adv Med Dent Sci Res 2020; 8(1):159-62. [ Google Scholar]
- Barua DR, Basavanna JM, Varghese RK. Efficacy of neem extract and three antimicrobial agents incorporated into tissue conditioner in inhibiting the growth of C albicans and S mutans. J Clin Diagn Res 2017; 11(5):ZC97-ZC101. doi: 10.7860/jcdr/2017/23784.9950 [Crossref] [ Google Scholar]
- Krishnamoorthy G, Narayana AI, Peralam PY, Balkrishanan D. To study the effect of Cocos nucifera oil when incorporated into tissue conditioner on its tensile strength and antifungal activity: an in vitro study. J Indian Prosthodont Soc 2019; 19(3):225-32. doi: 10.4103/jips.jips_387_18 [Crossref] [ Google Scholar]