Avicenna J Dent Res. 11(4):116-119.
doi: 10.34172/ajdr.2019.23
Original Article
Frequency of Different Types of Artifacts among Oral and Maxillofacial Histopathological Slides in Zanjan Dental School from 2015 to 2017
Sona Rafieyan 1  , Mahya Farsadeghi 2
, Mahya Farsadeghi 2  , Parsa Firoozi 3, Mehdi Sokhansanj 4, *
, Parsa Firoozi 3, Mehdi Sokhansanj 4, * 
Author information:
1Assistant Professor, Department of Oral and Maxillofacial Pathology, School of Dentistry, Zanjan University of Medical Sciences, Zanjan, Iran.
2Dentist, School of Dentistry, Zanjan University of Medical Sciences, Zanjan, Iran.
3Faculty of Dentistry, Department of Oral and Maxillofacial Surgery, Zanjan University of Medical Sciences, Zanjan, Iran.
4Dentist, Zanjan University of Medical Sciences, Zanjan, Iran.
Abstract
Background: Artifact refers to an artificial or replaced structure in histopathological slides as a result of an extraneous factor. Given the influence of identification and awareness of the types of artifacts on the correct diagnosis, the frequency of artifacts in oral and maxillofacial histopathological slides was assessed.
Methods: In this cross-sectional study, census method was used to assess 119 oral and maxillofacial histopathological slides retrieved from the archive of Zanjan Dental School from 2015 to 2017. Artifacts were divided into three groups arising from the surgeon’s performance, technician’s performance, and specimen transfer to the laboratory. Statistical analysis of data was performed using an independent t test in SPSS software version 18.0.
Results: The average numbers of artifacts arising from the surgeon’s performance, technician’s performance, and specimen transfer to the laboratory were 3.90±1.14, 3.08±1.10, and 0, respectively. The mean number of artifacts arising from the surgeon’s performance was significantly higher compared to the other two groups (P<0.01) and the most common ones included fragmentation, split, and tear. The most common artifacts arising from the technician’s performance were fold/wrinkle, chaffer, and floater. There was no artifact arising from specimen transfer to the laboratory.
Conclusions: The results indicated a high frequency of various artifacts in the studied slides. Therefore, paying more attention to slide preparation protocols and proficient performance during the biopsy procedure as well as further cooperation between the surgeon, pathologist, and laboratory technician can be useful in reducing the frequency of artifacts and achieving a better diagnosis.
Keywords: Artifacts, Biopsy, Oral pathology
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Rafieyan S, Farsadeghi M, Firoozi P, Sokhansanj M. Frequency of Different Types of Artifacts among Oral and Maxillofacial Histopathological Slides in Zanjan Dental School from 2015 to 2017 Avicenna J Dent Res. 2019;11(4):116- 119. doi: 10.34172/ajdr.2019.23.
Background
Highlights
-
The frequency of artifacts in oral and maxillofacial histopathological slides is high.
-
It should paying more attention to slide preparation protocols and proficient performance during the biopsy procedure
According to the American Academy of the Oral and Maxillofacial Pathology, any abnormal tissue removed from the oral and maxillofacial area should be sent to a pathologist for evaluation and diagnosis (1). Biopsy plays an undeniable role in forensic medicine (2). Therefore, a microscopic examination, the gold standard for most of the lesions, has been accepted as a principle (1,3).
Artifacts refer to artificial structures or tissue alterations on histopathological slides that had not been observed during tissue lifetime before the histopathologic examination. The presence of artifacts in histopathologic slides can lead to inaccurate diagnosis (4,5).
During tissue manipulation, a small amount of extra pressure via forceps, tight stitches, or blunt scalpels may lead to histopathological artifacts (6,7). Seify et al divided histopathological artifacts into three main categories: 1) Artifacts caused by surgeons 2) Those caused by technicians 3) Artifacts related to sample transferring. Moreover, they demonstrated that the most common artifact caused by surgeons is split, and the most common artifact caused by technicians is formalin pigmentation. They indicated that the appropriate performance of a surgeon, pathologist, and laboratory technician in terms of obtaining histopathological samples is necessary to reduce the artifacts (5). Shah et al showed split and folding as the most common artifacts observed in their study. They argued that a large number of artifacts might be produced during processing, microtomy, and staining procedures (4). Artifacts can change normal morphological and cytological characteristics which can lead to inaccurate diagnosis (4,8). Therefore, full knowledge of these artifacts is critical for taking preventive measures (9).
The current study aimed to investigate the frequency of artifacts in oral and maxillofacial histopathological slides retrieved from the archives of Zanjan Dental School from 2015 to 2017.
Materials and Methods
This descriptive cross-sectional study was conducted to investigate artifacts in all oral and maxillofacial histopathological slides retrieved from the archives of Department of the Oral and Maxillofacial Pathology of Zanjan Dental School.
Intact slides with undamaged labels were included in the study. While the exclusion criteria were the fracture of the slides and labels, as well as the separation of the labels from the slides. Moreover, repetitive slides from each block were excluded to avoid bias. Artifacts were classified into three groups: those caused by the surgeon’s performance, those caused by the technician’s performance, and those caused during the sample transfer to the laboratory. The artifacts caused by the surgeon’s performance include fragmentation, split, tear, crush, pseudocyst, hemorrhage, vacuolization, and starch (5,6) (Figure 1).

Figure 1.
Artifact Caused by the Technician’s Performance. A: Fragmentation, hematoxylin-eosin staining (×40), B: Split, hematoxylin-eosin staining (×100), C: Tear, hematoxylin-eosin staining (×100), D: Crush, hematoxylin-eosin staining (×100).
.
Artifact Caused by the Technician’s Performance. A: Fragmentation, hematoxylin-eosin staining (×40), B: Split, hematoxylin-eosin staining (×100), C: Tear, hematoxylin-eosin staining (×100), D: Crush, hematoxylin-eosin staining (×100).
Artifacts related to the technician’s execution includes fold/wrinkle, chaffer, floater, a tangential cut, bubble, formalin pigmentation, foreign body, thick section, and a residual wax (6,10). Autolysis is the only artifact that is usually caused by sample transferring (Figure 2) (5).
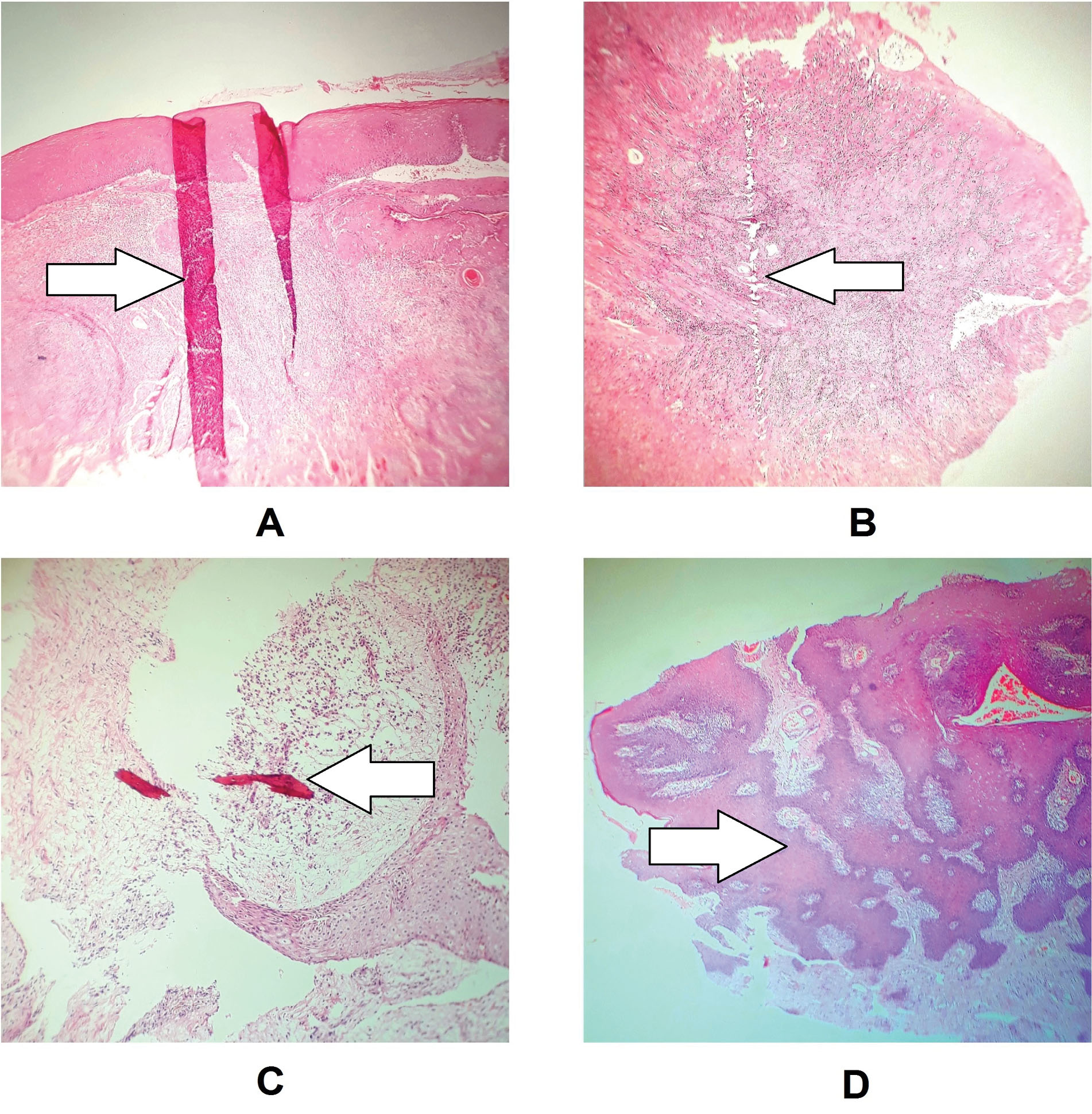
Figure 2.
Artifact Caused by the Technician’s Performance. A: Fold/wrinkle, hematoxylin-eosin staining (×40), B: Chaffer, hematoxylin-eosin staining (×40), C: Floater, hematoxylin-eosin staining (×100), D: Tangential cut artifact, hematoxylin-eosin staining (×40).
.
Artifact Caused by the Technician’s Performance. A: Fold/wrinkle, hematoxylin-eosin staining (×40), B: Chaffer, hematoxylin-eosin staining (×40), C: Floater, hematoxylin-eosin staining (×100), D: Tangential cut artifact, hematoxylin-eosin staining (×40).
For the slides evaluation, one of the authors (MS) examined the assessed slides which were re-evaluated by another researcher (SR) to ensure the validity of the results. Additionally, some slides examined by the author (MS) were evaluated and observed again by the same author after two weeks to ensure the reliability of results. Olympus Optical Microscope (MODEL CX21FS1C, Japan) was used for sample examination. The obtained data were imported into the SPSS software version 18.0, and the independent t test was used for data analysis. The frequency of the artifacts ranged from high to low.
The present study was not carried out on human or animal live samples, and the slides were prepared from the archive. Additionally, the information of patients, surgeons, and technicians were kept confidential.
Results
In this study, different histopathologic artifacts were studied in samples retrieved from the archives of the Department of Oral and Maxillofacial Pathology of Zanjan Dental School in 2017. The sample size after the exclusion of 27 slides was 119. The average number of artifacts caused by the surgeon’s performance, the technician’s performance, and the sample transfer to the laboratory were 1.14 ± 3.90, 1.10 ± 3.08, and 0, respectively. The mean number of artifacts caused by the surgeon’s performance was significantly higher than that of the other two groups (P < 0.01).
The most common artifacts caused by the surgeon’s performance were fragmentation (88.1%), split (84%), and tear (77.3%), and the most common artifacts caused by the technician’s performance were folding (88.2%), chaffer (57.1%) and floater (53.8%). There was no artifact caused by the sample transfer to the laboratory (Table 1).
Table 1.
Frequency of Different Artifacts in Histopathological Slides
|
|
Type of Artifact
|
Frequency of Artifacts (%)
|
| Artifacts caused by the surgeon’s performance |
Fragmentation |
106 (89.1) |
| Split |
100 (84) |
| Tear |
92 (77.3) |
| Crush |
64 (53.8) |
| Pseudocyst |
46 (38.7) |
| Hemorrhage |
39 (32.8) |
| Vacuolization |
18 (15.1) |
| Starch |
0 (0) |
| Artifacts caused by the technician’s performance |
Fold/Wrinkle |
105 (88.2) |
| Chaffer |
68 (57.1) |
| Floater |
64 (53.8) |
| Tangential cut |
52 (43.7) |
| Bubble |
35 (29.4) |
| Formalin pigmentation |
31 (26.1) |
| Foreign body |
8 (6.7) |
| Thick section |
3 (2.5) |
| Residual wax |
0 (0) |
| Autolysis |
0 (0) |
Discussion
The results of this study showed that 100% of the samples had at least one artifact caused by the surgeon’s performance or the technician’s performance. However, no artifacts were found in any of the slides due to the sample transfer to the laboratory.
Hemorrhage, extravasation, vacuolization of epithelium and connective tissue, and the separation of connective tissue layers are taken place by injection of an anesthetic agent into the biopsy region (11). Vacuolization was observed in approximately 15% of the samples. Hemorrhage resulting from the insertion of the needle into the vascular tissues is normal.
During tissue manipulation, the lowest pressure can lead to artifacts that appear in the form of crush, hemorrhage, split, fragmentation, and pseudocyst. These artifacts result from pressure of forceps, tight stitches, or a blunt scalpel blade (6,12). Seoane et al (12) studied 354 samples in 2004 to evaluate artifacts in oral biopsies prepared by general dentists and oral and maxillofacial surgeons. The most common artifacts caused by oral and maxillofacial surgeons were split, crush, hemorrhage, and fragmentation, respectively. Since the surgeon prepared the biopsies for slides in the current study, the results can be compared with the results of the surgeon’s performance in the study of Seoane et al. They are almost the same. Tissue may be torn by surgical forceps (13). Accordingly, a high percentage has been reported in other studies for this artifact (5). In this study, the tear was also observed as the third common artifact. Starch powder which is used as lubricant in surgical gloves can create artifacts by contaminating the specimen and mimic the appearance of atypical epithelial cells (6). In the present study, starch was not observed.
Artifacts caused by the technician’s performance may be created at each stage of the laboratory processes, such as tissue processing, embedding, or staining procedure (14). The formalin pigmentation is an artifact caused by the formalin fixation in acidic pH or environments with high temperature and humidity. Moreover, formalin pigmentation is more common in the tissues with high hemorrhage, and its frequency in the study of Saravani et al has been reported to be 90% (1), which is significant compared to the results of the present study. Microtomy is a tool used to cut the tissues for microscopic examination, and the formalin concentration, pollution, and long fixation time make it difficult to cut the samples. This issue can lead to some artifacts that occur in case of inappropriate technique including fold, chaffer, and alternate thick and thin sections (6,13). Chaffer is observed in the form of thin hollow bands, which is a result of scalpel vibration (2). Tangential cut of epithelium gives an impression of pseudoinvasion (4). In this study, a significant number of examined slides had this artifact. Contamination with the foreign body often makes it difficult to interpret the specimen, and we encounter it when paper, cotton gauze, or cork is used to prepare the tissue (13).
In this study, about 6% of the slides were affected by foreign body contamination. In a study by Sarwan et al (1), the most abundant artifacts caused by the technician’s performance were formalin pigmentation (90.9%), curling/folding (86.9%), and bubble (74.5%). The results of this study are in line with that of the current study. Although hemorrhage was not included in the three common groups of artifacts in the present study, its frequency is almost equal in both studies. In the artifacts caused by the technician, the frequency of the curling/folding is approximately the same as that of the present study. The floater can be formed as a result of the transfer of residual particles from the previous sections to the next section, which is attributed to irregular and improper cleaning of the sharp surfaces and water baths (6). In this study, a high frequency was observed for floater. The artifacts created during staining can be attributed to the artifact of the residual wax, which prevents the penetration of dye solution into the tissue, leaving areas completely devoid of stain (10). No residual wax was found in this study. After staining, in the mounting stage, in which the stained section is mounted on the slide, especially when the mounting medium is thin, the bubble formation under the slide is possible (6); this artifact influenced the samples of the present study.
Conclusions
The immediate and accurate fixation of the tissue sample is necessary to prevent autolysis and tissue damage and to stabilize the cellular proteins (2). The biopsy specimen should be placed in a large container with a sufficient amount of the 10% formalin solution for 24 hours (9,14). 10% formalin is considered as the best fixing agent to achieve optimal fixation. The amount of fixing agent should be 20 times more than the sample size (10). If the specimen is placed in solutions other than 10% formalin, tissue structures are lysed and affected which can prevent definite diagnosis (5). There were no cases of autolysis in this study.
Limitations
One of the limitations of this study is the lack of examination of all potential artifacts, which could be effective in estimating the mean number of artifacts caused by both the surgeon and technician. The results of this study indicate a high frequency of artifact types in the studied slides. Therefore, paying more attention to protocols for slide preparation, subtle execution during the biopsy process, as well as greater collaboration among surgeons, pathologists, and laboratory technicians, can help reduce the frequency of artifacts and achieve a better diagnosis.
Conflict of Interest Disclosures
None.
Acknowledgment
The authors would like to thank the Research Deputy of Zanjan University of Medical Sciences for financial support and the Department of Oral and Maxillofacial Pathology of the School of Dentistry that collaborated with this research.
Ethical Statement
This study was approved by the Ethics Committee of Zanjan University of Medical Sciences, Zanjan, Iran (Ethical code: ZUMS.REC.1396.90).
Authors’ Contributions
SR and OM contributed substantially to the conception and design of the study and the acquisition of data. MFS and PF provided critical revision of the article and provided final approval of the version to publish. MS as the corresponding author verifies that all individuals who made contributions to this study are either included as authors or are acknowledged at the end of the paper. The authors read and approved the final manuscript.
References
- Saravani S, Kadeh H, Shahsavari M, Shahrakipour M, Mosafer B. Evaluation of artifacts in oral and maxillofacial histopathological slides. Journal of Dentomaxillofacial Radiology, Pathology and Surgery 2016; 5(3):11-6. doi: 10.18869/acadpub.3dj.5.3.11 [Crossref] [ Google Scholar]
- Krishnanand PS, Kamath VV, Nagaraja A, Badni M. Artefacts in oral mucosal biopsies–a review. J Orofac Sci 2010; 2(1):57-62. [ Google Scholar]
- Yanduri S, Pandey G, Kumar V, Suma S, Madhura M. Artifacts in oral biopsy specimens: a comparison of scalpel, punch, and laser biopsies. Indian J Oral Health Res 2016; 2(2):100-5. doi: 10.4103/2393-8692.196147 [Crossref] [ Google Scholar]
- Shah A, Kulkarni M, Gabhane M. Artifacts in oral biopsies: a study. Indian J Stomatol 2012; 3(4):217-20. [ Google Scholar]
- Seify S, Mehdizadeh M, Bijani A, Alipour A, Nafarzadeh S. An investigation into the frequency and type of artifacts in oral histopathologic slides. J Res Dent Sci 2014; 10(4):260-5. [ Google Scholar]
- Kumar K, Shetty DC, Dua M. Biopsy and tissue processing artifacts in oral mucosal tissues. Int J Head Neck Surg 2012; 3(2):92-8. [ Google Scholar]
- Bindhu P, Krishnapillai R, Thomas P, Jayanthi P. Facts in artifacts. J Oral Maxillofac Pathol 2013; 17(3):397-401. doi: 10.4103/0973-029x.125206 [Crossref] [ Google Scholar]
- Choudhary S, Saxena A, Das A, Gulati G, Khare A, Prasad KD. Artefact & Classification. Saudi J Med Pharm Sci 2016; 2(6):141-5. doi: 10.21276/sjmps.2016.2.6.4 [Crossref] [ Google Scholar]
- Rastogi V, Puri N, Arora S, Kaur G, Yadav L, Sharma R. Artefacts: a diagnostic dilemma - a review. J Clin Diagn Res 2013; 7(10):2408-13. doi: 10.7860/jcdr/2013/6170.3541 [Crossref] [ Google Scholar]
- Rolls GO, Farmer NJ, Hall JB. Artifacts in Histological and Cytological Preparations. 1st ed. Melbourne: Leica Microsystems; 2008. p. 106.
- Kumaraswamy KL, Vidhya M, Rao PK, Mukunda A. Oral biopsy: oral pathologist’s perspective. J Cancer Res Ther 2012; 8(2):192-8. doi: 10.4103/0973-1482.98969 [Crossref] [ Google Scholar]
- Seoane J, Varela-Centelles PI, Ramírez JR, Cameselle-Teijeiro J, Romero MA. Artefacts in oral incisional biopsies in general dental practice: a pathology audit. Oral Dis 2004; 10(2):113-7. doi: 10.1111/j.1354-523x.2003.00983.x [Crossref] [ Google Scholar]
- Khan S, Tijare M, Jain M, Desai A. Artifacts in histopathology: a potential cause of misinterpretation. Research & Reviews: Journal of Dental Sciences 2014; 2(2):23-31. [ Google Scholar]
- Meghana S, Ahmedmujib B. Surgical artefacts in oral biopsy specimens: Punch biopsy compared to conventional scalpel biopsy. J Oral Maxillofac Pathol 2007; 11(1):11-4. doi: 10.4103/0973-029x.33957 [Crossref] [ Google Scholar]