Avicenna J Dent Res. 11(3):79-82.
doi: 10.34172/ajdr.2019.15
Original Article
Bilirubin in Saliva: A Potential Biomarker for Detecting Neonatal Jaundice
Mohammad Hosein Mirzaii Dizgah 1  , Mohammad Reza Mirzaii Dizgah 2, Iraj Mirzaii-Dizgah 3, *
, Mohammad Reza Mirzaii Dizgah 2, Iraj Mirzaii-Dizgah 3, *  , Hadi Lachinani 3, Banafsheh Dormanesh 4, Maryam Veisizadeh 5
, Hadi Lachinani 3, Banafsheh Dormanesh 4, Maryam Veisizadeh 5
Author information:
1Student Research Committee, School of Dentistry, AJA University of Medical Sciences; Tehran, Iran
2Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
3Department of Physiology, School of Medicine, AJA University of Medical Sciences, Tehran, Iran
4Department of Pediatrics, School of Medicine, AJA University of Medical Sciences, Tehran, Iran
5Department of Pediatrics, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Background: Precise and quick diagnosis of jaundice in the neonates is of major medical and economic importance. Biomarkers in serum are regularly applied in clinical settings. Nevertheless, saliva could be a substitute to assess biomarkers. In this study, we aimed to detect the probable association between salivary and serum levels of bilirubin in jaundiced neonates.
Methods: A case-control survey was performed on 30 healthy neonates and 30 neonates with jaundice hospitalized in Mirzakhochekkhan and Baharloo hospitals, Tehran, Iran. Bilirubin levels were assayed in serum and unstimulated whole saliva by a photometric method. Pearson correlation and Student’s t tests were used for statistical analysis.
Results: The mean salivary and serum levels of total bilirubin were significantly higher in the neonates with jaundice compared to healthy individuals. A moderate correlation was observed between serum and salivary concentrations of total bilirubin.
Conclusions: Serum and salivary levels of bilirubin are meaningfully correlated in jaundiced neonates. This correlation may facilitate the monitoring and evaluation of bilirubin levels non-invasively for jaundice.
Keywords: Bilirubin, Jaundice, Neonate, Saliva
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Mirzaii Dizgah MH, Mirzaii Dizgah MR, Mirzaii-Dizgah I, Lachinani H, Dormanesh B, Veisizadeh M. Bilirubin in Saliva: A Potential Biomarker for Detecting Neonatal Jaundice. Avicenna J Dent Res. 2019;11(3):79-82. doi: 10.34172/ ajdr.2019.15.
Background
Highlights
-
Unstimulated whole saliva bilirubin was significantly higher in the newborn suffer jaundice.
-
here was a moderate positive correlation between serum and saliva bilirubin in newborns.
-
The cut-off value of salivary bilirubin for the diagnosis of jaundice was 0.33 mg/dL.
Hyperbilirubinemia (very high level of bilirubin) causes neonatal jaundice which is one of the most common problems in the first week of birth and affects many term and preterm infants (1). Although hyperbilirubinemia is generally mild and of little clinical significance (2), the neonatal nervous system has the greatest impact on the toxic effects of bilirubin (3-5). Therefore, neonatal bilirubin level is measured to prevent the damage caused by hyperbilirubinemia and provide timely treatment.
Saliva, as compared to plasma, can be used to diagnose and evaluate disease progression. It is a biological fluid that can be collected easily, noninvasively, stress-free and several times without causing discomfort to patients compared to blood sampling (6,7). However, it is not usually used to diagnose diseases, like other biological samples. Saliva can be considered in the rapid diagnosis of a wide range of diseases. For example, research on saliva can be used to diagnose cancer, autoimmune disorders, kidney diseases, endocrine disorders, psychiatric disorders, infectious diseases, and dental diseases, as well as the severity of some diseases. Saliva can not only provide data about the severity of the disease and the body’s condition but also provide data that may not be available when using the serum test.
Up to the present time, nearly all the bilirubin assays are based on serum levels. However, blood collection from a vein is very difficult, injurious, could be a cause of infection, and requires a skilled person in newborns. Accordingly, the use of saliva as a harmless sampling method could be a reliable substitute.
Materials and Methods
The parents of all newborns registered in the study gave verbal informed consent. Thirty term neonates, who were hospitalized due to jaundice in the phototherapy department of Mirzakhochekkhan and Baharlou hospitals in Tehran, were recruited as the case group. The normal control group was comprised of 30 healthy term infants who had no evidence of jaundice.
The blood sample was collected from each newborn by venipuncture from 8 to 10 AM. The blood was centrifuged at 3000 g for about 5 minutes, and the serum was separated and frozen at −70°C until analysis.
The infants were not fed 1 hour before the saliva collection. The saliva samples (0.2 mL) were collected using a vacuum cleaner and frozen at −70°C until analysis.
The bilirubin value was measured using purchased kits by photometric methods (Pars Azmoon, Karaj, Iran).
The data are expressed as the mean ± standard error of the mean (SEM). Unpaired two-tailed Student’s t test, the Pearson correlation test, and receiver operating characteristic (ROC) analysis were used.
Results
As expected, serum total bilirubin level (mg/dL) was significantly higher in jaundice (13.04 ± 0.57) than control (7.03 ± 0.54) group (P = 0.001) (Figure 1a).
Unstimulated salivary level of total bilirubin (mg/dL) was significantly higher in the newborns suffered jaundice (0.48 ± 0.04) than in the controls (0.29 ± 0.03) (P = 0. 029) (Figure 1b). In saliva, the total bilirubin concentration was 1.5%-12% of that in the serum.
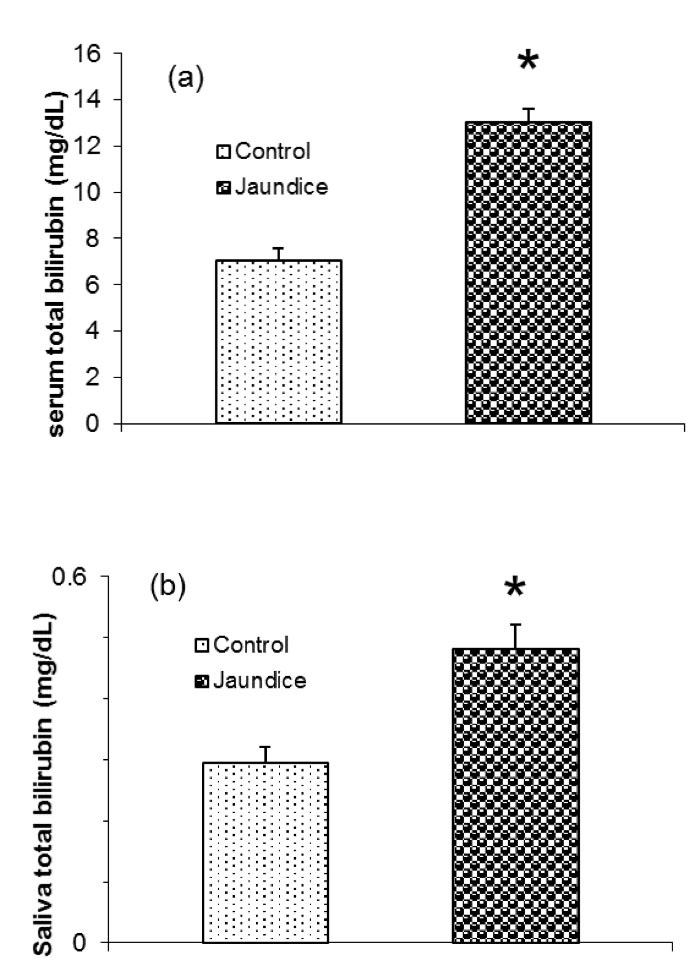
Figure 1.
Total Bilirubin Levels in Serum (a) and Unstimulated Saliva (b) in Jaundice and Healthy Newborns. *P < 0.05. Data are presented as means ± SEM.
.
Total Bilirubin Levels in Serum (a) and Unstimulated Saliva (b) in Jaundice and Healthy Newborns. *P < 0.05. Data are presented as means ± SEM.
There was a moderate positive association between serum and unstimulated salivary levels of total bilirubin in the newborn (r = 0. 472; P= 0.006).
The cut-off value of unstimulated salivary total bilirubin for the diagnosis of jaundice was 0.33 mg/dL (ROC-area under the curve = 0.835). With this cut-off, the sensitivity was 84%, and the specificity was 70%.
Discussion
This study aimed to measure and compare bilirubin levels in the saliva of healthy and jaundice neonates in order to find out whether the measure of this marker in saliva, due to its advantages over serum, can be an alternative medium for serum. In this study, we demonstrate that – in a small pilot study with 30 patients – the salivary level of total bilirubin, as well as the serum level of it, elevates in jaundice neonates as compared to control neonates and also the salivary level of bilirubin positively correlates with the serum level of it. To our knowledge, this is the first time that the salivary level of bilirubin in neonates who suffer jaundice has been evaluated.
Several pieces of evidence suggest that salivary tests have been continuously evaluated to diagnose, monitor, and predict disease prognosis. However, it has been revealed that some biochemical molecules can be assessed in the oral fluids of patients, for example, p53 (8), MMP-3 (9) and MMP-13 in oral lichen planus (10), cortisol and testosterone in xerostomia (11-13), cardiac troponin T, troponin I and cathepsin L in myocardial infarction (14-16), oral glucose tolerance test in diabetes mellitus (17), aminotransaminase in hypoxia (18), and calcium and magnesium in giardiasis (19).
Some studies have been conducted on the presence of markers in the saliva of neonates, such as bile acid, cortisol, and erythropoietin in various diseases (20-22). Saliva also may well serve as an alternative matrix for therapeutic drug monitoring of caffeine in patients with apnea and methylphenidate in patients with attention-deficit/hyperactivity disorder (23,24). The results of this study showed that bilirubin is detectable in the saliva of neonates.
Jaundice can occur when excessive bilirubin levels accumulate in the blood. Bilirubin is a natural product of red blood cell decomposition, which is usually metabolized in the liver but is often metabolized slowly in infants because their liver is still not fully functional. It is estimated that about 84% of term neonates experience some degrees of jaundice in their first week of life (2).
The presence of high levels of bilirubin in plasma for a long time can cause permanent damage to the central nervous system. However, the effects of severe hyperbilirubinemia in the newborn baby can be eliminated through follow-up, diagnosis, and appropriate treatment (25). Therefore, it is essential to screen newborns for hyperbilirubinemia. The measurement of serum bilirubin is the most frequent laboratory test in the well-infant nursery. However, blood sampling is an invasive, stressful, and time-consuming procedure which requires skilled health personal and can also lead to infections. Therefore, a good correlation between serum and salivary levels of total bilirubin in neonates with clinical jaundice would be very useful to reduce the side effects of blood sampling. To substitute saliva for serum in biological tests, there should be a high association between the level of parameters measured in plasma and saliva (26). There was a moderate association between serum and salivary concentrations of bilirubin in this study. However, salivary levels of bilirubin may reflect its serum concentration.
There were some limitations to this study. Due to the lack of hospital’s capacities to do this test for a large number of infants and the presence of few subjects, the results cannot be generalized to the general population, and no decision can be drawn about the salivary cut-off level of total bilirubin.
Conclusions
Serum and salivary levels of bilirubin are meaningfully correlated in jaundiced neonates. This correlation may facilitate the monitoring and non-invasive evaluation of bilirubin levels for jaundice.
Conflict of Interest Disclosures
The authors declared that they have no conflict of interests.
Acknowledgements
We would like to thank the personnel of Mirzakhochekkhan and Baharlou hospitals and the infants’ parents for their cooperation.
Ethical Statement
The present study was approved by the Ethics Committee of AJA University of Medical Sciences (No:84/90/405). Written informed consent was obtained from all the parents in the study.
Authors’ Contribution
All the authors have contributed to the conception and design of the study. MRMD and MHMD contributed to data collection and drafted the manuscript. The statistical analyses and interpretation of data were carried out by IMD. All the authors have read and approved the final manuscript.
References
- Zhu J, Xu Y, Zhang G, Bao Y, Wu M, Du L. Total serum bilirubin levels during the first 2 days of life and subsequent neonatal morbidity in very low birth weight infants: a retrospective review. Eur J Pediatr 2012; 171(4):669-74. doi: 10.1007/s00431-011-1634-z [Crossref] [ Google Scholar]
- Bhutani VK, Stark AR, Lazzeroni LC, Poland R, Gourley GR, Kazmierczak S. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr 2013; 162(3):477-82. doi: 10.1016/j.jpeds.2012.08.022 [Crossref] [ Google Scholar]
- Shapiro SM. Bilirubin toxicity in the developing nervous system. Pediatr Neurol 2003; 29(5):410-21. doi: 10.1016/j.pediatrneurol.2003.09.011 [Crossref] [ Google Scholar]
- Watchko JF, Maisels MJ. Jaundice in low birthweight infants: pathobiology and outcome. Arch Dis Child Fetal Neonatal Ed 2003; 88(6):F455-8. doi: 10.1136/fn.88.6.f455 [Crossref] [ Google Scholar]
- Olds C, Oghalai JS. Bilirubin-induced audiologic injury in preterm infants. Clin Perinatol 2016; 43(2):313-23. doi: 10.1016/j.clp.2016.01.006 [Crossref] [ Google Scholar]
- Hofman LF. Human saliva as a diagnostic specimen. J Nutr 2001; 131(5):1621S-5S. doi: 10.1093/jn/131.5.1621S [Crossref] [ Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I. Serum and saliva magnesium in postmenopausal women with xerostomia. Climacteric 2012; 15(5):496-9. doi: 10.3109/13697137.2011.624212 [Crossref] [ Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I, Miri-Zarandi N. Unstimulated salivary p53 in patients with oral lichen planus and squamous cell carcinoma. Acta Med Iran 2015; 53(7):439-43. [ Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I, Mahboobi N, Shirazian S, Harirchi I. Serum and saliva MMP-3 in patients with OLP and oral SCC. J Contemp Dent Pract 2015; 16(2):107-11. doi: 10.5005/jp-journals-10024-1645 [Crossref] [ Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I. Serum and saliva collagenase-3 (MMP-13) in patients with oral lichen planus and oral squamous cell carcinoma. Med J Islam Repub Iran 2015; 29:218. [ Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I, Mirjalili N. Relationship of unstimulated saliva cortisol level with severity of oral dryness feeling in menopausal women. Aust Dent J 2011; 56(2):171-4. doi: 10.1111/j.1834-7819.2011.01320.x [Crossref] [ Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I, Mirjalili N. Relationship of stimulated whole saliva cortisol level with the severity of a feeling of dry mouth in menopausal women. Gerodontology 2012; 29(1):43-7. doi: 10.1111/j.1741-2358.2010.00403.x [Crossref] [ Google Scholar]
- Agha-Hosseini F, Moosavi MS, Mirzaii-Dizgah I. Salivary flow, testosterone, and femur bone mineral density in menopausal women with oral dryness feeling. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 115(5):612-6. doi: 10.1016/j.oooo.2012.11.014 [Crossref] [ Google Scholar]
- Mirzaii-Dizgah I, Riahi E. Salivary high-sensitivity cardiac troponin T levels in patients with acute myocardial infarction. Oral Dis 2013; 19(2):180-4. doi: 10.1111/j.1601-0825.2012.01968.x [Crossref] [ Google Scholar]
- Mirzaii-Dizgah I, Riahi E. Salivary troponin I as an indicator of myocardial infarction. Indian J Med Res 2013; 138(6):861-5. [ Google Scholar]
- Mirzaii-Dizgah I, Riahi E. Serum and saliva levels of cathepsin L in patients with acute coronary syndrome. J Contemp Dent Pract 2011; 12(2):114-9. doi: 10.5005/jp-journals-10024-1019 [Crossref] [ Google Scholar]
- Mirzaii-Dizgah MH, Mirzaii-Dizgah I, Mirzaii-Dizgah MR. Oral glucose tolerance test in unstimulated saliva of healthy individuals. European J Gen Dent 2016; 5(1):15-8. doi: 10.4103/2278-9626.172736 [Crossref] [ Google Scholar]
- Mominzadeh M, Mirzaii-Dizgah I, Mirzaii-Dizgah MR, Mirzaii-Dizgah MH. Stimulated saliva aminotransaminase alteration after experiencing acute hypoxia training. Air Med J 2014; 33(4):157-60. doi: 10.1016/j.amj.2014.03.004 [Crossref] [ Google Scholar]
- Shaddel M, Mirzaii-Dizgah I, Sharifi-Sarasiabi K, Kamali Z, Dastgheib M. Stimulated and unstimulated saliva levels of calcium and magnesium in giardiasis. Biol Trace Elem Res 2017; 179(1):8-12. doi: 10.1007/s12011-017-0943-0 [Crossref] [ Google Scholar]
- Hedenborg G, Norlander A, Norman A. Bile acids in serum, ultrafiltrate of serum and saliva from patients with cholestatic jaundice. Scand J Clin Lab Invest 1987; 47(1):83-9. [ Google Scholar]
- Hung GY, Jeng MJ, Lin CY, Soong WJ, Hwang B. The relationship between serum and saliva erythropoietin concentrations in adults, full-term and premature infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1998; 39(6):380-5. [ Google Scholar]
- El-Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva - are our assays good enough?. Ann Clin Biochem 2017; 54(3):308-22. doi: 10.1177/0004563216687335 [Crossref] [ Google Scholar]
- Lee TC, Charles BG, Steer PA, Flenady VJ. Saliva as a valid alternative to serum in monitoring intravenous caffeine treatment for apnea of prematurity. Ther Drug Monit 1996; 18(3):288-93. doi: 10.1097/00007691-199606000-00012 [Crossref] [ Google Scholar]
- Preiskorn J, Studer S, Rauh R, Lukačin R, Geffert C, Fleischhaker C. Interindividual and intraindividual variation of methylphenidate concentrations in serum and saliva of patients with attention-deficit/hyperactivity disorder. Ther Drug Monit 2018; 40(4):435-42. doi: 10.1097/ftd.0000000000000520 [Crossref] [ Google Scholar]
- Neocleous C, Adramerina A, Limnaios S, Symeonidis S, Spanou C, Malakozi M. A comparison between transcutaneous and total serum bilirubin in healthy-term greek neonates with clinical jaundice. Prague Med Rep 2014; 115(1-2):33-42. doi: 10.14712/23362936.2014.4 [Crossref] [ Google Scholar]
- Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta 2007; 383(1-2):30-40. doi: 10.1016/j.cca.2007.04.011 [Crossref] [ Google Scholar]