Avicenna J Dent Res. 11(2):66-71.
doi: 10.34172/ajdr.2019.12
Original Article
Color Change of Primary Teeth Following Using 4 Types of Iron Supplements Available in the Iranian Pharmacopeia
Soudeh Tayebi 1  , Mohammad Esmaeilzade 2, Loghman Soufi Rezai 3, Farnoush Fotovat 4, *
, Mohammad Esmaeilzade 2, Loghman Soufi Rezai 3, Farnoush Fotovat 4, *  , Roya Najafi Vosogh 5, Niloufar Faregh 6
, Roya Najafi Vosogh 5, Niloufar Faregh 6
Author information:
1Assistant Professor, Department of Pediatrics, Faculty of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
2Pedodontist, Tehran, Iran
3Associated Professor, Department of Operative and Aesthetic Dentistry, Faculty of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
4Assistant Professor, Department of Prosthodontics, Faculty of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
5PhD Candidate, Department of Biostatistics, Faculty of Health, Hamadan University of Medical Sciences, Hamadan, Iran 6 Dentist, Hamadan, Iran
6Dentist, Hamadan, Iran
Abstract
Background: Iron supplementation plays an important role in the growth and development of children. However, iron causes persistent discoloration of primary teeth, which creates some concerns for the parents. This study aimed to assess the color change of the primary teeth following the use of four types of iron supplements available in the Iranian pharmacopeia.
Methods: In this in vitro experimental study, 60 primary incisors (120 tooth surfaces) with intact crowns were collected and randomly divided into 5 groups (1 control and 4 experimental groups). The color of the teeth was then measured at baseline (time 0) and 24, 48, 72, and 96 hours after the immersion in solutions containing 250 mL of artificial saliva in the control group and artificial saliva plus iron supplements containing 100 mg of iron in the experimental groups using the Vita Easy Shade Compact. Finally, the data were analyzed using the ANOVA test and pairwise comparisons were made using Tukey’s and least significant difference tests via SPSS, version 23.
Results: The primary teeth showed a significant color change after 24 and 48 hours of immersion in the solutions (P<0.05) but no significant change was noted after 72 and 96 hours of immersion (P>0.05).
Conclusions: In general, the color change of the primary teeth was not significantly different following exposure to the four iron supplements. Eventually, the Iranian and foreign-made iron supplements caused a similar color change in the teeth.
Keywords: Dentition, Primary, Tooth Discoloration, Iron, Dietry
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Tayebi S, Esmaeilzade M, Soufi Rezai L, Fotovat F, Najafi Vosogh R, Faregh N. Color Change of Primary Teeth Following Using 4 Types of Iron Supplements Available in the Iranian Pharmacopeia. Avicenna J Dent Res. 2019;11(2):66- 71. doi: 10.15171/ajdr.2019.12.
Background
Highlights
Adequate nutrition plays a major role in physical growth and development. In addition, nutritional requirements may vary depending on body size, physical activity, and health status. Iron is considered as an important element that is required by the human body (1) and is found in all viable tissues including the enamel, dentin, and dental pulp (2-4). Children also need iron for growth and development, as well as the synthesis of red blood cells. The highest amount of iron in the human body is found in hemoglobin present in red blood cells, which has the important task of carrying oxygen from the lungs to the tissues (4-6).
Further, iron supplements in Iran are often in the form of iron drops. However, tooth discoloration is a common complication of iron drop consumption (3,7-9). Edible iron compounds available in the Iranian market are mainly in the form of ferrous sulfate, which has a bad taste and causes the black discoloration of the primary teeth (4,10) and is unpleasant for the children and parents (11,12).
It is believed that the extrinsic staining of the teeth or dental plaque, which is highly prevalent in children taking iron supplements, has a metallic origin (13). Iron uptake is less by the sound surfaces of the primary teeth following the use of iron drops, and therefore, less discoloration occurs on such surfaces. However, by an increase in the surface energy of the teeth due to the development of white spot lesions or caries on enamel surfaces, the amount of iron uptake and the magnitude of discoloration would increase accordingly (14).
On the other hand, the iron inhibits the progression of dental caries since it increases enamel resistance. Furthermore, iron salts decrease acid production by oral bacteria, as well as the count of Streptococcus mutans in the dental biofilm (13,15).
The staining of the primary teeth is regarded as a common concern of parents since it can decrease social communications and the self-esteem of the children due to the dark, unaesthetic appearance of the teeth. Such discolorations can also be mistaken for caries. Similarly, the lack of knowledge about such discolorations is a common cause of frequent dental visits of parents to pediatric dentists (2,7,14,16,17).
The three main characteristics of the color include the hue, chroma, and value (18). Several methods are available for the assessment of the degree of tooth discoloration following staining. In the present study, the Vita Easy Shade Compact (DEASYCBU, Germany) was used for this purpose (2).
In addition, 4 commonly used types of iron supplements available in the Iranian pharmacopeia were evaluated, the details of which are provided in Table 1.
Table 1.
Composition of the understudy products.
|
Name
|
Ingredient/1 mL
|
Company/Country
|
Other Properties
|
| Ferbolin |
125 mg of ferrous sulfateheptahydrate |
Shahr Daru, Sharyar, Iran |
Contains 1.5 mg of sodium saccharine |
| Ferrodrop |
125 mg of ferrous sulfate heptahydrate |
DonyayeBehdasht, Sharyar, Iran |
Contains 1.5 mg of sodium saccharine and 0.01 mg of ascorbic acid |
| Irovit |
75 mg of ferrous sulfate |
Vitane, Germany |
Favorable taste |
| Feroglobin |
10 mg of ferrous sulfate |
Vitabiotics, UK |
contains vitamin B1, B2, B6, B12, C, folic acid, zinc, iron, copper, and calcium |
Iron drops are routinely prescribed for children by pediatric physicians. However, the resultant discoloration of the primary teeth is of great concern for the parents and leads to their frequent dental visits. In some cases, the parents discontinue iron drop consumption by their children since they believe that it causes dental caries while it is critical for the growth and development of their children. Thus, to find an iron supplement with the minimal discoloration of the primary teeth, this study aimed to compare the color change of the primary teeth following utilizing four commonly used types of iron drops that are available in the Iranian pharmacopeia.
Materials and Methods
In this in vitro, experimental study, 4 iron supplements were evaluated, including Ferbolin (ShahrDarou, Sharyar, Iran), Ferrodrop (DonyayeBehdasht, Sharyar, Iran), Irovit (Vitane, Germany), and Feroglobin (Vitabiotics, UK). Moreover, 60 extracted sound primary incisors (120 surfaces) were collected from different pediatric dental clinics in Hamadan and stored in artificial saliva. The teeth were cleaned by the pumice paste (Rotec GmbH and Company, KG, Germany) and prophy brush and sectioned mesiodistally. All teeth were then mounted in acrylic resin and the entire tooth surface was coated with nail varnish except for a square-shaped window at the middle third of the crown measuring 2x2 mm. The color of the teeth was measured using the Vita Easy Shade Compact (DEASYCBU, USA)
The sample size was calculated to be 24 teeth in each group (a total of 120 teeth) according to a previous study by Shabzendehdar et al (12) and assuming type one error of 5% and the power of 90%.
Next, the teeth were randomly divided into 5 groups (1 control and 4 experimental groups). The control samples were immersed in 250 mL of the artificial saliva. Additionally, the samples in the experimental groups were immersed in solutions containing 250 mL of artificial saliva and iron supplements containing 100 mg of the pure iron (6.6 mL, 4 mL, 4 mL, and 6.6 mL of Irovit, Ferbolin, Ferrodrop, and Feroglobin, respectively). The teeth were removed from the solutions after 24, 48, 72, and 96 hours (2) and rinsed with artificial saliva. The tooth color was measured using the Vita Easy Shade Compact (DEASYCBU, USA). In addition, the fresh solutions were prepared and the teeth were immersed in fresh solutions. The color of teeth was measured again at the designated time points, followed by determining the amount of the color change (∆E) using the following formula (1).
The color of the teeth was determined by measuring a*, L*, and b* parameters in CIE Lab color space. The L*, a*, and b* indicate darkness/lightness, redness (+)/greenness (-), and yellowness (+)/blueness (-), respectively (19).
The amount of ∆E>1 refers to a difference in the color which is detectable by a minimum of 50% of the observers. Further, ∆E>3.7 is defined as a clinically unacceptable color change (19).
The normal distribution of data and the homogeneity of variances were evaluated using the Kolmogorov-Smirnov and Levene tests, respectively. In the case of the normal distribution of the data and the equality of variances, the color change in each group at 24, 48, 72, and 96-hour time points was analyzed using the ANOVA test. On the other hand, the non-parametric Kruskal-Wallis test was applied in the case of the non-normal distribution of data and the inequality of the variances. Furthermore, the Tukey test, the least significant difference (LSD) test, independent t-test, and the Mann-Whitney test were used for pairwise comparisons.
Moreover, the repeated measures ANOVA was applied to assess the color change of the teeth at the four-time points. To use this test, the normal distribution of data was evaluated using the Kolmogorov-Smirnov test and the sphericity assumption was evaluated using the Mauchly test. Finally, the Friedman test was applied if the assumptions were violated, followed by using the Tukey’s and LSD tests for pairwise comparisons.
Results
Given that the color change data in the Feroglobin group did not have a normal distribution at 24 hours, the Kruskal-Wallis test was used for the comparison of a color change among the 5 groups, which showed a significant difference in this regard (P < 0.05). Likewise, the independent t-test was then used for pairwise comparisons. As shown in Table 2, significant differences were observed between the control and Irovit, control and Ferbolin, as well as control and Ferrodrop groups (P < 0.05). However, the difference between Irovit and Ferbolin, Irovit and Ferrodrop, and finally, Ferbolin and Ferrodrop groups was not significant (P> 0.05).
Table 2.
Results of the independent t test for the pairwise comparisons of groups regarding the color change at 24 hours
|
Groups
|
P Value
|
| Irovit-control |
0.001 |
| Ferbolin-control |
0.0001 |
| Ferrodrop-control |
0.001 |
| Irovit-Ferbolin |
0.460 |
| Irovit-Ferrodrop |
0.214 |
| Ferrodrop-Ferbolin |
0.303 |
The results of the Mann-Whitney test (Table 3) revealed a significant difference in the color change between the control and Feroglobin, as well as Irovit and Feroglobin groups (P < 0.05). On the other hand, no significant difference was found between Ferbolin and Feroglobin, as well as Ferrodrop and Feroglobin groups (P > 0.05).
Table 3.
Results of the Mann-Whitney Test for the Pairwise Comparisons of Groups Regarding the Color Change at 24 Hours
|
Groups
|
P Value
|
| Control-Feroglobin |
0.010 |
| Irovit-Feroglobin |
0.027 |
| Ferbolin-Feroglobin |
0.050 |
| Ferrodrop-Feroglobin |
0.353 |
Considering that both assumptions were met, the ANOVA test was applied to compare the groups at 48 hours, indicating a significant difference in the color change of the five groups at 48 hours (P < 0.05). Contrarily, no significant difference was noted in this regard among the five groups at 72 and 96 hours (P > 0.05).
Table 4 shows the mean and standard deviation (SD) of the color change in the 5 groups at different time points. At 24 and 72 hours, the minimum color change was observed in the control group. Among the experimental groups, the minimum color change was noted in the Ferbolin whereas the maximum color change was found in the Feroglobin group. At 48 hours, the minimum color change was noted in the control group. Among the experimental groups, the minimum color change was detected in the Irovit group while the maximum color change was noted in the Feroglobin group. Finally, the minimum color change was observed in the control group at 96 hours. Among the experimental groups, the minimum color change was noted in the Ferbolin whereas the maximum color change was found in the Ferrodrop group.
Table 4.
Descriptive Results of the Color Change in the Five Groups at Different Time Points
|
|
Time (h)
|
|
|
24
|
48
|
72
|
96
|
|
|
Mean (SD)
|
Mean (SD)
|
Mean (SD)
|
Mean (SD)
|
| Control group |
7.7688 (3.17586) |
7.7688 (3.17586) |
7.7688 (3.17586) |
7.7688 (3.17586) |
| Ferbolin group |
8.4167 (3.19805) |
9.7125 (3.24242) |
10.9708 (3.19354) |
12.6792 (3.20176) |
| Irovit group |
8.6292 (2.46673) |
9.6667 (2.43162) |
11.1250 (2.08874) |
12.9042 (2.23130) |
| Ferrodrop group |
9.4042 (3.36833) |
10.6583 (3.26802) |
11.5083 (3.25308) |
13.4125 (2.77384) |
| Feroglobin group |
10.1750 (2.84899) |
11.0000 (2.87750) |
12.2333 (2.47591) |
13.3042 (2.51905) |
|
P value |
0.0001 |
0.003 |
0.167 |
0.837 |
Note. SD: Standard deviation.
The mean (SD) of the color change of the teeth at 0, 24, 48, 72, and 96 hours revealed that the color of the primary teeth remained the same at all time points in the control group.
The results demonstrated that long-term use of iron supplements caused a greater color change considering the normal distribution of the data at all time points in the Irovit, Ferbolin, and Ferrodrop groups and the mean color change (Table 4) at different time points. The repeated measures ANOVA showed a significant difference in the color at 0, 24, 48, 72, and 96 hours in the Irovit, Ferbolin, and Ferrodrop groups (P < 0.05). Additionally, the pairwise comparisons by the LSD test represented significant differences in this respect at different time points (P=0.0001).
Table 4 presents the mean (SD) of the color change of the teeth at 0, 24, 48, 72, and 96 hours in the Feroglobin group. The results indicated a greater color change in the long-term use of the Feroglobin. Based on the results of the Kolmogorov-Smirnov test, the data were not normally distributed in this group at 96 hours. Thus, the Friedman test was applied, which showed a significant difference in the color change of the teeth in the Feroglobin group at different time points (P < 0.05). Therefore, pairwise comparisons were made using the Wilcoxon test, which revealed significant differences in all pairwise comparisons (P=0.0001).
Figure 1 illustrates the mean ∆E in the 5 groups at different time points.
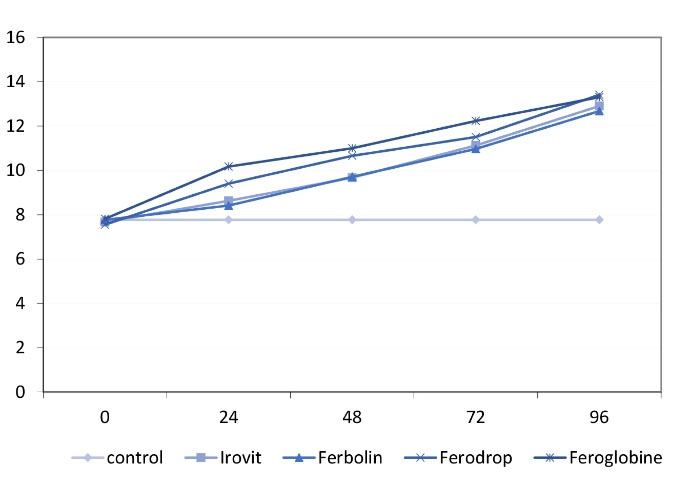
Figure 1.
The Mean Color Change in the Five Groups at Different Time Points.
.
The Mean Color Change in the Five Groups at Different Time Points.
Discussion
Physical and mental development is a dynamic process. In addition, adequate nutrition plays a fundamental role in physical growth and development although nutritional requirements may vary relying on the body size, physical activity, and health status. Iron is an essential element required by the human body (16) and is found in all viable tissues. Similarly, iron has a confirmed role in the growth and development, as well as the synthesis of the red blood cells and the transfer of oxygen from the lungs to the tissues (2-6). Further, a significant correlation was reported between iron deficiency and dental caries (8,13,19-23). Furthermore, iron supplements in Iran are mainly prescribed as the iron drops. Tooth discoloration is a major complication of using iron drops. On the other hand, edible iron compounds available in the Iranian market are mainly in the form of ferrous sulfate, which has a bad taste and leads to the black discoloration of the primary teeth that is unpleasant for the children and parents (11,15).
Tooth discoloration following iron supplementation may be due to superficial stains, also known as metallic stains, which can be easily removed or may penetrate deep into the tooth structure, leading to a persistent color change, which is not easily removable.
In general, iron leaves brown stains but the iron drops cause black stains due to the deposition of the iron sulfide (24,25).
Color is composed of red, green and blue lights and color perception depends on the following 3 factors (18): (I) the observer, (II) the object and (III) the light source. Any change in any of these three factors can alter the color perception.
Evidence shows greater tooth discoloration following iron supplementation in areas with demineralized or porous enamel due to caries. Iron can be washed off from the outermost enamel surface thus it appears that this element is physically absorbed by the tooth structure and causes discoloration associated with the biofilm formation (3).
Considering the high prevalence of tooth discoloration following iron supplementation and the parents’ concerns in this respect, which may lead to the discontinuation of the use, the present study aimed to find an iron supplement with the minimal discoloration of the primary teeth among four commonly used types of iron drops that are available in the Iranian pharmacopeia.
In this study, 60 extracted primary anterior teeth (120 tooth surfaces) with intact crowns were collected and randomly classified into five groups (one control and four experimental groups). The color of the teeth was measured at baseline (time 0) and 24, 48, 72, and 96 hours after the immersion in solutions containing 250 mL of artificial saliva in the control group and artificial saliva plus iron supplements containing 100 mg of the iron in the experimental groups using the Vita Easy Shade Compact. This device has high accuracy and reliability for color measurement. The other advantages of this device include high precision, the reproducibility of measurements, the provision of objective data, the easy use, the direct transfer of data, and cost-effectiveness (26,27). The results showed that the teeth exposed to Ferbolin for 96 hours experienced less discoloration compared to the other iron supplements. Ferrodrop caused the maximum color change. At 96 hours, the color change increased in the experimental groups but it was not significant. The mean ∆E in Ferrodrop, Feroglobin, Irovit and Ferbolin groups was 5.86, 5.49, 5.24, and 4.91 at 96 hours, respectively.
Some previous studies assessed the effect of iron supplementation on the color of primary teeth. For example, Pani et al (2) compared the discoloration potential of the primary teeth exposed to two types of iron syrups, namely, ferrous fumarate (FF) and ferric oxide polymaltose (FOP). They found that using a combination of different forms of iron decreased the severity of staining compared to the equivalent dose of each iron supplement. In addition, FOP caused greater discoloration than FF. The mean ∆E in FF plus FOP, FF, and FOP groups changed by two, three, and four units after 72 hours, respectively. Our findings are in line with the results of the above-mentioned study since the mean ∆E increased in both studies. The mean ∆E was not significantly different among the groups in our study while this difference was significant in the above-mentioned study. Finally, the assessment time points, the device used for color assessment, and the methodology were the same in both studies but different iron supplements were evaluated in our study compared to theirs (2).
Similarly, Mehran et al (16) evaluated the effect of two types of iron drops on the color of the primary teeth and greater than that in the teeth subjected to Fer-in-Sol iron drop. The results of their study indicated an increase in ∆E over time, which corroborates with the results of our study. Both studies used ferrous sulfate iron supplements as well. The mean ∆E was not significantly different among the groups in our study while this difference was significant in the above study, which may be due to the difference in other ingredients present in ferrous sulfate iron drops, methodology, and the method of color assessment in the two studies. In the study by Mehran et al, a restorative dentist evaluated the color change visually. They also used ICP (Vista Pro, Australia) to assess the atomic absorption rate. Eventually, they evaluated structural changes using a scanning electron microscope (16).
In another study, Makarem et al (14) compared the color change of the primary enamel following the use of iron drops and showed that the iron drop manufactured by Mashhad School of Pharmacy caused less color change in the primary enamel. The mean ∆E increased over time in both studies. Further, ferrous sulfate supplements were used in both studies. However, the mean ∆E demonstrated no significant difference among the groups in our study while this difference was significant in their study, which is probably due to the difference in other ingredients present in ferrous sulfate iron drops, methodology, and method of color assessment in the studies. In their study, half of the samples were etched with 37% phosphoric acid and the color change was determined applying the scanning electron microscopy.
Considering that the iron compound was ferrous sulfate in all four evaluated supplements in our study, less color change caused by Ferbolin compared to the other supplements may be attributed to the absence of vitamin C because this vitamin has an erosive property and causes enamel porosity. As the result, the surface energy and the iron uptake by the enamel surface represents an increase. Eventually, the results showed that Feroglobin, Irovit, and Ferrodrop containing vitamin C caused a greater color change.
Conclusions
In general, the color change of the primary teeth was not significantly different following exposure to the four iron drops and the Iranian and foreign-made iron supplements led to similar color changes.
Authors’ Contribution
Soudeh Tayebi:Study concept-study design,article design
Mohammad Esmaeilzade: Study concept-study design
Loghman Soufi Rezai: article design
Farnoush Fotovat::Manuscript preparation,Editing and review
Roya Najafi Vosogh :statistical analysis
Niloufar Faregh :Laboratory procedure
Ethical Statement
This retrospective study was approved by the Ethics Committee of Hamadan University of Medical Sciences ((Ethical approval code: IR.UMSHA.REC.1396.689)).
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
References
- Mehran M, Mohammadi Bassir M, Jafari SJJDM. Effect of two kinds of iron drops on the discoloration, atomic absorption and structural changes of primary teeth enamel. J Dent Med 2009; 21:290-9. [ Google Scholar]
- Pani SC, Alenazi FM, Alotain AM, Alanazi HD, Alasmari AS. Extrinsic tooth staining potential of high dose and sustained release iron syrups on primary teeth. BMC Oral Health 2015; 15:90. doi: 10.1186/s12903-015-0072-0 [Crossref] [ Google Scholar]
- Stangel I, Valdes E, Xu J. Absorption of iron by dentin: its role in discoloration. J Biomed Mater Res 1996; 31(2):287-92. doi: 10.1002/(SICI)1097-4636(199606)31:2<287::AIDJBM17>3.0.CO;2-I. [Crossref] [ Google Scholar]
- Wen X, Paine ML. Iron deposition and ferritin heavy chain (Fth) localization in rodent teeth. BMC Res Notes 2013 Jan 2; 6:1. doi: 10.1186/1756-0500-6-1 [Crossref] [ Google Scholar]
- Anderson GJ, Vulpe CD. Mammalian iron transport Cell Mol Life Sci. 2009 Oct; 66(20):3241-61. doi: 10.1007/s00018-009-0051-1 [Crossref] [ Google Scholar]
- Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochim Biophys Acta 2012; 1820(3):188-202. doi: 10.1016/j.bbagen.2011.10.013 [Crossref] [ Google Scholar]
- De-Regil LM, Jefferds ME, Sylvetsky AC, Dowswell T. Intermittent iron supplementation for improving nutrition and development in children under 12 years of age. Cochrane Database Syst Rev.2011 Dec 7;(12):CD009085.10.1002/14651858.CD009085.pub2.
- Eshghi A, Kowsari-Isfahan R, Rezaiefar M, Razavi M, Zeighami S. Effect of iron containing supplements on rats’ dental caries progression. J Dent (Tehran) 2012; 9(1):14-9. [ Google Scholar]
- Wu TW, Tsai FP. Comparison of the therapeutic effects and side effects of oral iron supplements in iron deficiency anemia. Drug Res (Stuttg) 2016; 66(5):257-61. doi: 10.1055/s-0035-1569326 [Crossref] [ Google Scholar]
- Dean JA. McDonald and Avery’s Dentistry for the Child and Adolescent-E-Book. Elsevier Health Sciences; 2015.
- Addy M, Moran J. Extrinsic tooth discoloration by metals and chlorhexidine II Clinical staining produced by chlorhexidine, iron and tea. Br Dent J 1985; 159(10):331. doi: 10.1038/sj.bdj.4805722 [Crossref] [ Google Scholar]
- Makarem A, Orafai H, Shabzendehdar M, Khashayarmanesh Z, Ebrahimzadeh SJJoDMUoMS. Comparsion of primary enamel discoloration caused by the use of three different iron drops 2006;30:247-54.
- Okazaki M, Takahashi J, Kimura H. Iron uptake of hydroxyapatite. J Osaka Univ Dent Sch 1985; 25:17-24. [ Google Scholar]
- Makarem A, Orafai H, Shabzendehdar M, Khashayarmanesh Z, Ebrahimzadeh S. Comparsion of primary enamel discoloration caused by the use of three different iron drops. Journal of Mashhad Dental School 2006; 30:247-54. [ Google Scholar]
- Royston E. The prevalence of nutritional anaemia in women in developing countries: a critical review of available information. World Health Stat Q 1982; 35(2):52-91. [ Google Scholar]
- Eskandarian T, Motamedifar M Hekmatfar S, Tamaddon AM. Comparison of the Effect of Three Types of Iron Drops on Surface Roughness of Deciduous Teeth in a Simulated Cariogenic Environment. Journal of Dental School 2013; 31(1):15-22. [ Google Scholar]
- Sintes JL, Miller SA. Influence of dietary iron on the dental caries incidence and growth of rats fed an experimental diet. Arch Latinoam Nutr 1983; 33(2):322-38. [ Google Scholar]
- Shillingburg HT, Sather DA, Wilson EL, Cain JR, Mitchell DL, Blanco LJ, et al. Fundamentals of Fixed Prosthodontics. Quintessence Publishing Company; 2012.
- Koumpia E, Athanasiou AE, Eliades T, Knosel M. Precision of reflectance spectrophotometer in measuring anterior tooth color. Open Dent J 2018; 12:884-895. [ Google Scholar]
- Charytan C, Bernardo MV, Koch TA, Butcher A, Morris D, Bregman DB. Intravenous ferric carboxymaltose versus standard medical care in the treatment of iron deficiency anemia in patients with chronic kidney disease: a randomized, active-controlled, multi-center study. Nephrol Dial Transplant 2013 Apr; 28(4):953-64. doi: 10.1093/ndt/gfs528 [Crossref] [ Google Scholar]
- Hassani AS, Amirmozafari N, Ordouzadeh N, Hamdi K, Nazari R, Ghaemi A. Volatile components of Camellia sinensis inhibit growth and biofilm formation of oral strepto. Pak J Biol Sci 2008; 11(10):1336-41. doi: 10.3923/pjbs.2008.1336.1341 [Crossref] [ Google Scholar]
- Tolentino K, Friedman JF. An update on anemia in less developed countries. Am J Trop Med Hyg 2007; 77(1):44-51. [ Google Scholar]
- Miguel JC, Bowen WH, Pearson SK. Effects of frequency of exposure to iron-sucrose on the incidence of dental caries in desalivated rats. Caries Res 1997; 31(3):238-43. doi: 10.1159/000262406 [Crossref] [ Google Scholar]
- Avery DR, McDonald RE. Dentistry for the Child and Adolescent. Mosby; 2004.
- Newman MG,Takei H, Klokkevold PR, Carranza FA. Clinical Periodontology. 13th ed. Elsevier; 2019.
- Rood JP, Shehab BA. The radiological prediction of inferior alveolar nerve injury during third molar surgery. Br J Oral Maxillofac Surg 1990; 28(1):20-5. doi: 10.1016/0266-4356(90)90005-6 [Crossref] [ Google Scholar]
- Zenthöfer A, Cabrera T, Corcodel N, Rammelsberg P, Hassel AJ. Comparison of the Easyshade Compact and Advance in vitro and in vivo. Clin Oral Investig 2014; 18(5):1473-9. doi: 10.1007/s00784-013-1118-9 [Crossref] [ Google Scholar]