Avicenna J Dent Res. 16(4):210-217.
doi: 10.34172/ajdr.1879
Original Article
Therapeutic Targeting of Akt1 in Oral Squamous Cell Carcinoma Using Natural Cinnamic Acids
Setareh Shojaei 1  , Amirhoseyn Naiini 1
, Amirhoseyn Naiini 1  , Shokoofeh Jamshidi 1
, Shokoofeh Jamshidi 1  , Amir Taherkhani 2, 3, *
, Amir Taherkhani 2, 3, * 
Author information:
1Department of Oral and Maxillofacial Pathology, School of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
2Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
3Research Center for Molecular Medicine, Institute of Cancer, Avicenna Health Research Institute, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Background: Akt1, a serine/threonine kinase, plays a central role in cancer development and progression. Its overexpression correlates with aggressive phenotypes and poor prognosis in several types of cancers, such as oral squamous cell carcinoma (OSCC). Cinnamic acid derivatives (CADs) from natural sources exhibit anticancer properties, making them potential Akt1 inhibitors.
Methods: The binding affinities of 19 CADs to the Akt1 catalytic cleft were evaluated using molecular docking simulations and then compared with the Akt1 inhibitor capivasertib. Interaction modes were analyzed to identify critical residues involved in ligand binding.
Results: Cynarin demonstrated the highest binding affinity (ΔGbinding =-13.46 kcal/mol, Ki=136.48 pM), forming three hydrogen bonds with Akt1. Rosmarinic acid with six hydrogen bonds also exhibited potent inhibition (ΔGbinding =-11.51 kcal/mol, Ki=3.67 nM). Both compounds represented superior binding compared to capivasertib.
Conclusion: Cynarin and rosmarinic acid from natural sources showed promising inhibitory potential against Akt1, suggesting their therapeutic values as anticancer agents targeting the PI3K/Akt pathway in OSCC.
Keywords: Akt1, Cinnamic acid, Cynarin, Molecular docking, Neoplasm, Rosmarinic acid
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Shojaei S, Naiini A, Jamshidi S, Taherkhani A. Therapeutic targeting of Akt1 in oral squamous cell carcinoma using natural cinnamic acids. Avicenna J Dent Res. 2024; 16(4):210-217. doi:10.34172/ajdr.1879
Background
Akt1, a serine/threonine protein kinase within the PI3K/Akt signaling pathway, is central in regulating cellular survival, proliferation, and growth. Its dysregulation has been linked to the development and progression of various malignancies, including oral squamous cell carcinoma (OSCC) (1-3). Notably, Akt1 overexpression significantly correlates with aggressive phenotypes and unfavourable prognoses in OSCC patients, highlighting its potential as a target for therapeutic interventions (1,4). In OSCC, Akt1 overexpression is associated with enhanced tumor growth, invasive potential, and resistance to programmed cell death (apoptosis) (1,2). Akt1 exerts its oncogenic influence by modulating various downstream targets, particularly the GSK-3β/β-catenin pathway, which is crucial for cell cycle progression, apoptosis evasion, and metastatic spread (5). Furthermore, the interaction between Akt1 and other oncogenic factors, such as CIP2A, underscores its role in perpetuating malignant phenotypes by reinforcing pro-tumorigenic signaling cascades (5). Preclinical studies in OSCC models suggest promising outcomes for Akt1 inhibition as a therapeutic strategy. Various small molecules and natural compounds identified through computational screening potently inhibit Akt1. These findings generally support the potential of targeting Akt1 for therapeutic benefit in OSCC. In addition, microRNAs (miRNAs) have been shown to modulate tumor radiosensitivity, primarily through their interaction with the PI3K/AKT pathway-a master regulator pathway of cancer cell survival and proliferation-along with other signaling cascades such as mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases, nuclear factor-κB, and transforming growth factor-β (6,7).
Recently, there has been a surge of interest in natural products as potential therapeutic agents. A diverse array of bioactive compounds derived from natural sources are currently undergoing preclinical and clinical evaluation to treat various diseases (8). These compounds hold significant promise for cancer therapy due to their multifaceted mechanisms of action. They can exert cytotoxic effects, induce apoptosis, inhibit angiogenesis and metastasis, arrest the cell cycle, and modulate cancer-associated molecular pathways. Natural products have the potential to synergistically augment the efficacy of conventional cancer treatments while mitigating associated side effects, ultimately leading to improved patient outcomes. Notably, several studies have highlighted the potential for these compounds to enhance the effectiveness of existing therapies while reducing their adverse effects (9-15).
Cinnamic acid and its derivatives, a class of naturally occurring aromatic carboxylic acids (16), are widely distributed in various plant sources, including Cinnamomum cassia, Panax ginseng, fruits, whole grains, vegetables, and honey (8,17). A substantial body of research has established the diverse biological activities of cinnamic acid derivatives (CADs), encompassing antioxidant, antimicrobial (18), anticancer (19), neuroprotective, anti-inflammatory, and antidiabetic properties (20). Notably, the promising role of CADs in cancer therapy has been extensively investigated, with their mechanisms of action primarily focusing on chemosensitization, inhibition of cellular proliferation, induction of apoptosis, and potentiation of the efficacy of conventional chemotherapeutic drugs (21-25).
Molecular docking, a computational technique employed in bioinformatics, plays a critical role in the preclinical phase of drug discovery. This methodology allows for the systematic investigation of ligand-receptor interactions, facilitating the prioritization of promising drug candidates from vast chemical libraries. This approach is precious due to drug development’s complex and resource-intensive nature. The process can take over a decade and incur costs exceeding $1 billion, with success rates for novel drug approvals remaining below 15% (26).
Given the established role of Akt1 in cancer development and the documented effectiveness of cinnamic acids in cancer treatment, this study explores the potential of cinnamates as Akt1 inhibitors. To investigate this hypothesis, molecular docking analysis is employed to assess the binding affinity of various CADs to the Akt1 catalytic cleft. The results are compared to those of established Akt1 inhibitors. It is expected that these findings offer valuable insights for developing novel therapeutic agents targeting Akt1 in cancer treatment.
Methods
Structural Preparation of Molecules
For this investigation, the crystal structure of Akt1 (Protein Data Bank [PDB] ID: 4GV1, resolution: 1.49 Å) was achieved from the Research Collaboratory for Structural Bioinformatics PDB (https://www.rcsb.org/). This file contained a single polypeptide chain (chain A) with 340 residues. Swiss-PdbViewer (version 4.1.0) was used to perform energy minimization on the enzyme structure to ensure its most stable conformation for subsequent analysis. Discovery Studio Visualizer (DSV) software was utilized to examine the interactions between the known inhibitor capivasertib (PubChem ID: 25227436) and the active site residues, identifying key residues within the Akt1 active site. This analysis highlighted Leu156, Gly157, Gly162, Val164, Ala177, Met227, Ala230, Glu234, Glu278, Asn279, and Met281 as critical for ligand binding.
Next, 19 CADs, mainly derived from fruits, vegetables, and medicinal plants, were selected as potential Akt1 inhibitors. The binding affinities of these compounds to the Akt1 catalytic cleft were assessed using molecular docking and then compared to the established inhibitor capivasertib, serving as a positive control. Consistent with previous studies, all small molecules underwent energy minimization using HyperChem software. Polar hydrogens were added to prepare 4GV1 for molecular docking, and Kollmann charges were assigned. The protein structure was further processed to include rotational freedom and local charges for the small molecules. These steps were conducted using MGLTools. Finally, the Protein Data Bank Quick Preparation Tool was employed to generate the necessary files for docking simulations, which were executed through the Cygwin64 Terminal.
Molecular Docking and Interaction Analyses
Docking simulations were performed using AutoDock (version 4.0) on a standard Windows PC with an Intel Core i5 processor, 8 GB RAM, and a 64-bit operating system. The semi-flexible docking method was used to calculate the Gibbs free energy of binding (ΔGbinding) in kcal/mol between cinnamic acids, capivasertib, and the Akt1 active site. A grid box of 60 × 60 × 60 Å was centered at -20.556 Å, 5.617 Å, and 11.523 Å, with a spacing of 0.375 Å for the analysis. The Lamarckian genetic algorithm generated 50 conformations per compound to assess binding affinities between the tested compounds, the control inhibitor, and the Akt1 active site. Docking results were clustered based on a 2.0 Å root mean square tolerance, selecting the conformation with the most negative ΔGbinding value from the most populated cluster for further analysis. DSV software visualized the interaction modes, including possible H-bonds, hydrophobic, and electrostatic interactions between the ligands and the Akt1 binding site.
Results
Binding Affinities
Our findings revealed that cynarin exhibits the most substantial inhibitory effect on Akt1 among the investigated compounds. It demonstrated a picomolar range of enzymatic activity constraint, with a binding affinity characterized by an inhibition constant (Ki) value of 136.48 pM and a ΔGbinding score of -13.46 kcal/mol. Rosmarinic acids also emerged as potent inhibitors, with a ΔGbinding score of -11.51 kcal/mol and a Ki value of 3.67 nM. These two compounds were selected for further investigation due to their high binding affinity, reflected by ΔGbinding values below -10 kcal/mol, suggesting a robust interaction with the target enzyme 4GV1.
Capivasertib represented a ΔGbinding score of -9.89 kcal/mol with the Akt1 enzyme. Accordingly, cynarin and rosmarinic acid illustrated a greater affinity for the Akt1 active site compared to the reference drug. A detailed analysis of the binding energies and Ki for all tested compounds, including the positive control inhibitor and the 4GV1 target enzyme, is provided in Table 1. Additionally, Figure 1 compares the ΔGbinding value of capivasertib with the superior binding affinities observed for the identified CADs.
Table 1.
Binding Energies and Inhibition Constant Ki Values Quantified for the Interactions Between the Akt1 Enzyme, 19 Cinnamic Acid Derivatives, and the Reference Inhibitor Capivasertib
|
PubChem ID
|
Ligand Name
|
Intermolecular Energy (kcal/mol)
|
Internal Energy (kcal/mol)
|
Torsional Free Energy (kcal/mol)
|
Unbound System’s Energy (kcal/mol)
|
Free Energy of Binding (kcal/mol)
|
K
i
|
| 6124212 |
Cynarin |
-7.94 |
-13.86 |
6.26 |
-2.08 |
-13.46 |
136.48 pM |
| 5281792 |
Rosmarinic acid |
-8.72 |
-8.28 |
4.47 |
-1.02 |
-11.51 |
3.67 nM |
| 1794427 |
Chlorogenic acid |
-5.09 |
-9.96 |
4.18 |
-1.54 |
-9.34 |
141.34 nM |
| 5281759 |
Caffeic acid 3-glucoside |
-7.9 |
-6.13 |
3.88 |
-1.21 |
-8.95 |
275.62 nM |
| 5372945 |
N-p-Coumaroyltyramine |
-11.1 |
-0.77 |
2.98 |
-0.17 |
-8.72 |
404.85 nM |
| 11380911 |
Cinnamyl caffeate |
-9.2 |
-2.07 |
2.39 |
-0.51 |
-8.37 |
730.39 nM |
| 5919576 |
Benzyl caffeate |
-8.05 |
-2.04 |
1.49 |
-0.62 |
-7.98 |
1.41 uM |
| 160355 |
Roscovitine |
-8.19 |
-3.5 |
2.68 |
-1.07 |
-7.92 |
1.55 uM |
| 5281787 |
Phenethyl caffeate |
-8.83 |
-1.77 |
2.39 |
-0.53 |
-7.68 |
2.34 uM |
| 637540 |
o-coumaric acid |
-7.2 |
-1.37 |
1.79 |
-0.06 |
-6.71 |
12.02 uM |
| 5472440 |
Artepillin C |
-8.48 |
-0.64 |
2.09 |
-0.35 |
-6.68 |
12.67 uM |
| 445858 |
Ferulic acid |
-7.72 |
-0.91 |
2.09 |
-0.23 |
-6.32 |
23.46 uM |
| 689043 |
Caffeic acid |
-7.0 |
-1.6 |
2.09 |
-0.19 |
-6.31 |
23.63 uM |
| 689075 |
Methyl caffeate |
-6.66 |
-1.38 |
1.49 |
-0.26 |
-6.29 |
24.32 uM |
| 637542 |
p-coumaric acid |
-7.31 |
-0.09 |
1.79 |
-0.03 |
-5.59 |
80.55 uM |
| 637775 |
Sinapinic acid |
-7.15 |
-1.29 |
2.39 |
-0.52 |
-5.53 |
87.96 uM |
| 6440361 |
Drupanin |
-6.95 |
-0.11 |
1.49 |
-0.08 |
-5.48 |
96.16 uM |
| 444539 |
Cinnamic acid |
-6.39 |
-0.13 |
1.49 |
-0.05 |
-4.97 |
226.58 uM |
| 819020 |
2-Methylcinnamic acid |
-5.48 |
-0.13 |
0.89 |
-0.13 |
-4.58 |
435.70 uM |
| 25227436 |
Capivasertib (Ctrl + ) |
-10.14 |
-2.75 |
2.39 |
-0.62 |
-9.89 |
55.94 nM |
Note. Ki: Inhibition constant; Akt1: Serine/threonine protein kinase within the PI3K/Akt signaling pathway; Ctrl: Control.
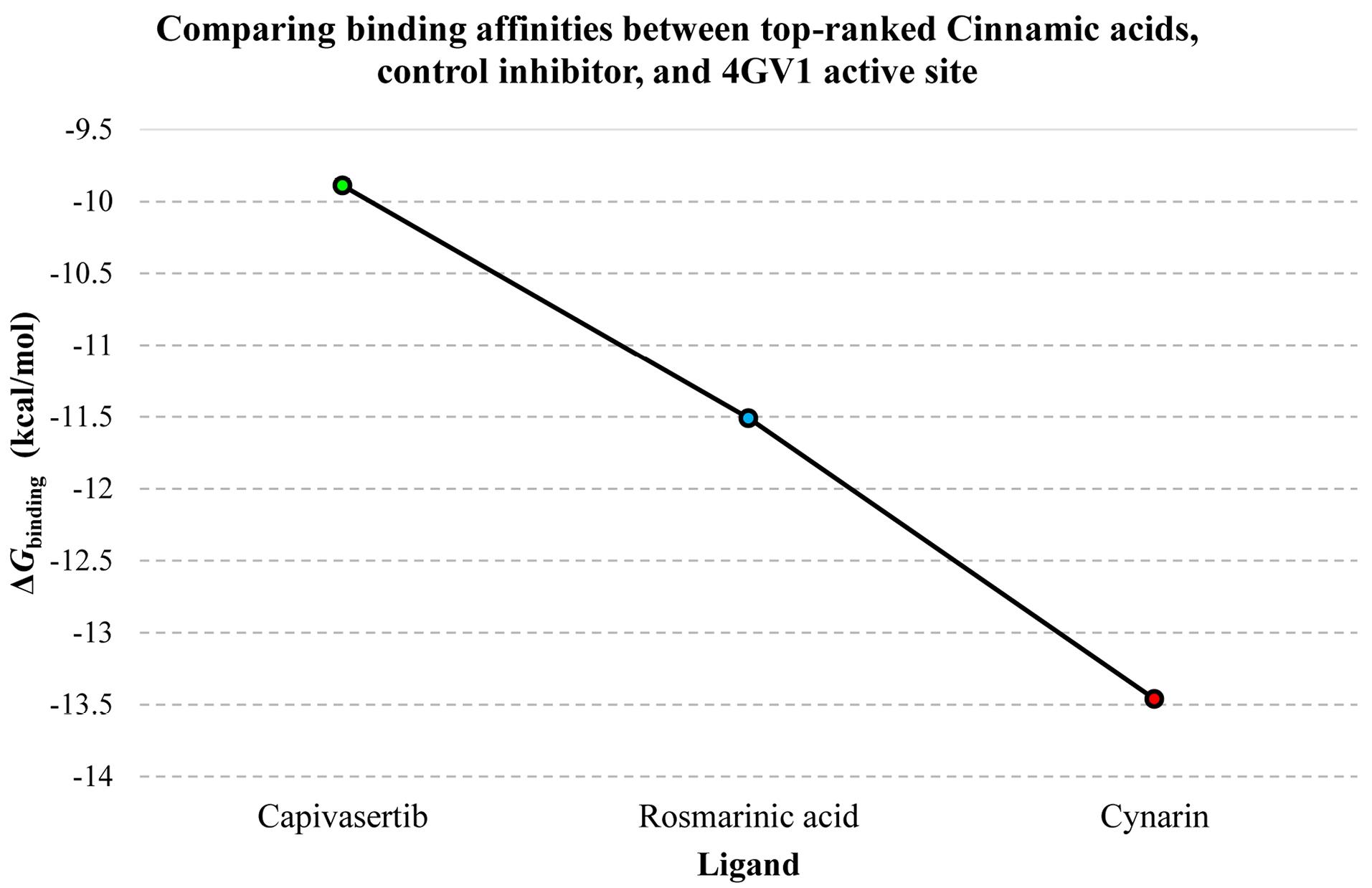
Figure 1.
The ΔGbinding Values Denoting the Gibbs Free Energy of Binding, Quantified in kcal/mol, Among the Catalytic Cleft of the Akt1 Enzyme, its Control Inhibitor, and the Top-Ranked Cinnamic Acid Derivatives. Note. The ligand designations are represented along the X-axis, while the corresponding Gibbs free binding energy is depicted on the Y-axis. Akt1, a serine/threonine protein kinase, is a crucial component of the PI3K/Akt signaling cascade
.
The ΔGbinding Values Denoting the Gibbs Free Energy of Binding, Quantified in kcal/mol, Among the Catalytic Cleft of the Akt1 Enzyme, its Control Inhibitor, and the Top-Ranked Cinnamic Acid Derivatives. Note. The ligand designations are represented along the X-axis, while the corresponding Gibbs free binding energy is depicted on the Y-axis. Akt1, a serine/threonine protein kinase, is a crucial component of the PI3K/Akt signaling cascade
Interaction Modes
A meticulous investigation of the interactions between cynarin, rosmarinic acid, capivasertib, and critical amino acid residues within the active site of the 4GV1 enzyme was undertaken using DSV software. Cynarin formed three hydrogen bonds, absent of any hydrophobic or electrostatic interactions. It is important to note that hydrogen bonds exceeding a distance of 5 Å were excluded from the study. Rosmarinic acid demonstrated the most hydrogen bonds, forming a total of six. This compound exhibited four hydrophobic interactions, and one was classified as miscellaneous. The control molecule, capivasertib, displayed two hydrogen bonds and seven hydrophobic interactions with residues within the enzyme’s catalytic domain. The reference drug also formed one electrostatic interaction and one miscellaneous interaction. Table 2 comprehensively enumerates these interactions and their corresponding distance details. Figure 2 displays two- and three-dimensional depictions of these compounds within the receptor’s active site.
Table 2.
Binding Modes Among the Catalytic Cavity of the 4GV1 Enzyme, the Top-Ranked Cinnamic Acid Derivatives, and Capivasertib, Employed as a Positive Control Inhibitor
|
Ligand Name
|
Hydrogen Bond (Distance in Å)
|
Hydrophobic Interaction (Distance in Å)
|
Electrostatic (Distance in Å)
|
Miscellaneous (Distance in Å)
|
| Cynarin |
Glu234 (4.54), Asp274 (4.30), and Phe161 (3.55) |
Na |
Na |
Na |
| Rosmarinic acid |
Met281 (4.54), Glu234 (4.68, 4.99, 4.68,), Gly157 (4.02), and Glu278 (3.58) |
Met281 (7.09), Ala177 (5.70), Leu156 (5.41), and Val164 (4.06) |
Na |
Met281 (5.26) |
| Capivasertib (Ctrl) |
Asn279 (3.54) and Glu278 (3.65) |
Phe438 (7.20), Leu156 (5.83), Val164 (4.90, 5.44), Ala177 (7.36, 5.40), and Met281(6.42) |
Glu234 (5.10) |
Met281 (6.06) |
Note. 4GV1 is a serine/threonine protein kinase within the PI3K/Akt signaling pathway; Ctrl: Control.
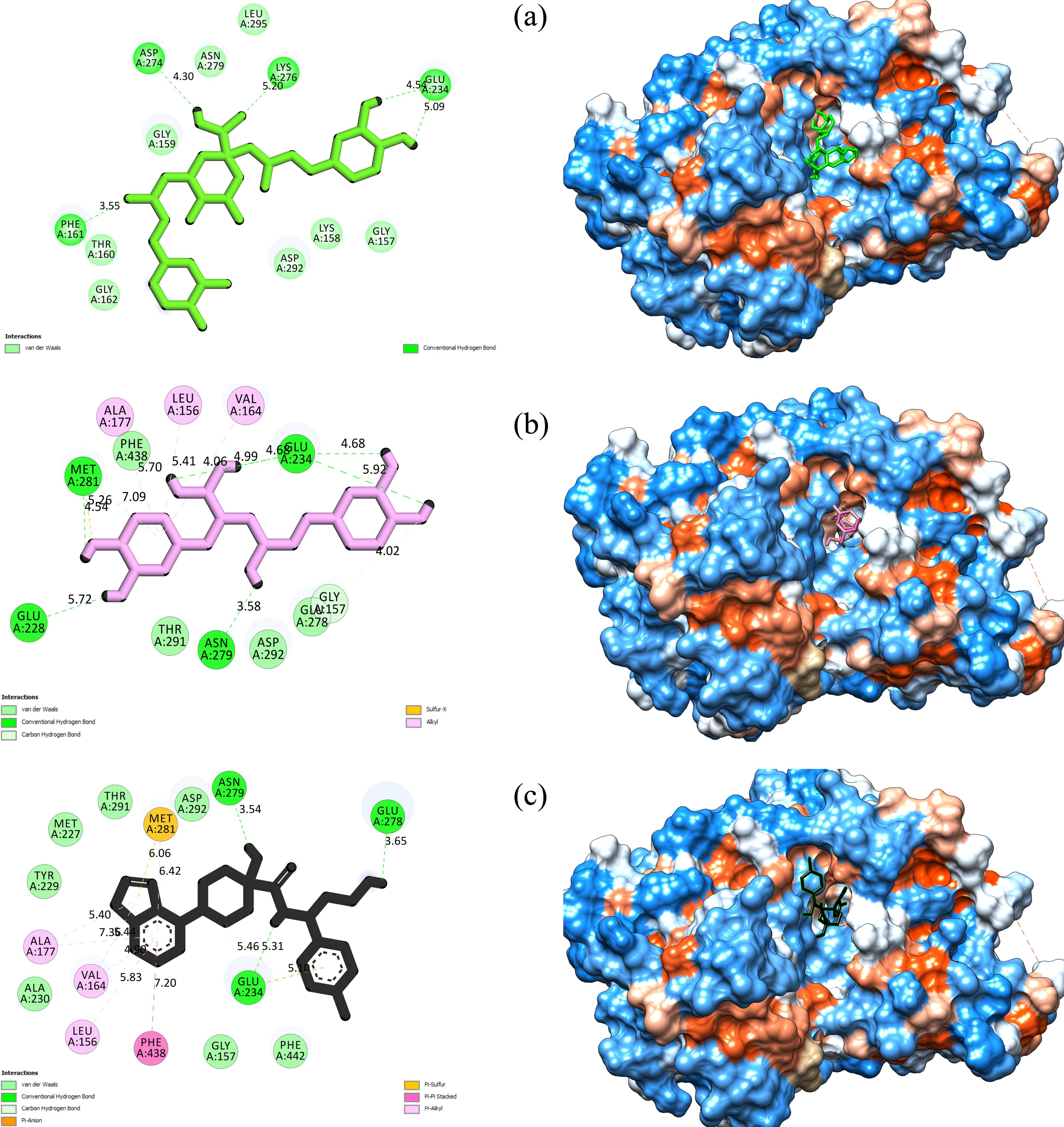
Figure 2.
Two- and Three-Dimensional View of (a) Cynarin, (b) Rosmarinic Acid, and (c) Capivasertib Within the Akt1 Active Site. Note. Act1 is a serine/threonine protein kinase within the PI3K/Akt signaling pathway
.
Two- and Three-Dimensional View of (a) Cynarin, (b) Rosmarinic Acid, and (c) Capivasertib Within the Akt1 Active Site. Note. Act1 is a serine/threonine protein kinase within the PI3K/Akt signaling pathway
Discussion
Akt1 exerts critical regulatory control over various cellular processes, including metabolism, proliferation, cell survival, growth, and blood vessel formation. Its aberrant activation is a prevalent characteristic observed in the initiation and advancement of malignant tumors, solidifying its status as a promising target for therapeutic intervention (3,27,28). This protein has promoted cancer development through various mechanisms, such as inhibiting programmed cell death, accelerating cellular proliferation, and facilitating metabolic adaptations within cancer cells (3,28). The upregulation of the PI3K/Akt/mTOR signaling pathway has been demonstrably linked to resistance to radiation therapy, a cornerstone treatment for many cancers (27). The inhibition of Akt1 and other components of this pathway has shown potential in increasing the sensitivity of cancer cells to radiation therapy, suggesting that a combined approach could potentially translate into improved patient outcomes (27).
Furthermore, Akt1’s critical role in cancer metastasis is highlighted by its involvement in cell migration and invasion, two essential steps in the metastatic process. Akt1’s influence on these processes is mediated through the remodeling of the cellular skeleton and the epithelial-to-mesenchymal transition, which is indispensable for cancer cells to acquire invasive and metastatic properties (3). The dysregulation of Akt1, therefore, not only contributes to the growth of the primary tumor but also facilitates the spread of cancer cells to distant organs, emphasizing its profound significance in cancer progression (3,28).
Research examining CADs as Akt pathway modulators encompasses comprehensive investigations integrating in silico molecular docking approaches with experimental validation studies. Among these derivatives, chlorogenic acid has emerged as a particularly significant compound, drawing substantial attention for its neuroprotective properties. In a pivotal study, Xiong et al (29) employed network pharmacology and molecular docking analyses to identify Akt1 as a central molecular target in chlorogenic acid-mediated tumor necrosis factor signaling regulation. Their findings, validated using a lipopolysaccharide-induced neuroinflammation mouse model and BV-2 cell lines, demonstrated that chlorogenic acid attenuates cognitive dysfunction by suppressing Akt-dependent microglial M1 polarization. In the context of diabetes therapeutics, Ramorobi et al (30) explored potential synergistic interactions between zinc and p-coumaric acid. Their molecular docking analyses revealed that the zinc-p-coumaric acid complex exhibits enhanced binding affinity for Akt, correlating with improved glucose uptake in cellular and tissue models. This finding suggests a novel therapeutic approach for diabetes management through Akt pathway modulation.
This study utilized a computational drug discovery approach to assess the inhibitory potential of various well-established cinnamic acids against Akt1. The obtained results were then subjected to a comparative analysis with capivasertib, a recognized reference drug for Akt1 inhibition. The computational analysis yielded promising results, with cynarin and rosmarinic acid demonstrating significant binding affinity to the Akt1 active site. The ΔGbinding value of capivasertib with 4GV1 was -9.89 kcal/mol. This value served as a benchmark for evaluating the binding affinity of other compounds to Akt1. Cynarin emerged as the most potent Akt1 inhibitor within the investigated cinnamic acids, boasting an impressive ΔGbinding value of -13.46 kcal/mol and a Ki value of 136.48 pM. Rosmarinic acid followed closely behind, exhibiting a binding energy of -11.51 kcal/mol and a Ki value of 3.67 nM, solidifying its potential as a promising Akt1 inhibitor.
Cynarin, a primary bioactive component isolated from artichoke (Cynara scolymus L.), has garnered significant scientific interest due to its diverse therapeutic potential. Research suggests that cynarin possesses noteworthy anti-inflammatory, antioxidant, and anticancer properties, likely mediated through various cellular mechanisms (31). Cynarin’s ability to inhibit human carbonic anhydrases is particularly notable, specifically CA VI. This suggests its potential application in managing conditions such as cancer and metabolic disorders (31). Additionally, computational simulations have identified cynarin as a potent inhibitor of MAPK3, a crucial regulator in cell proliferation and apoptosis pathways. This inhibitory effect on MAPK3 proposes a potential mechanism by which cynarin may exert anticancer effects, paving the way for novel cancer treatment strategies (32). Molecular docking and dynamics simulations have provided further insight into cynarin’s potential as an inhibitor of matrix metalloproteinase-9 (MMP-9). MMP-9 is an enzyme implicated in the degradation of the extracellular matrix and associated with various diseases, including cancer. This indicates another potential mechanism for cynarin’s anticancer properties (33). Zhang et al (34) confirmed the antitumor effects of cynarin alongside chlorogenic and caffeic acids isolated from the burdock root. Their study revealed a positive correlation between these compounds and tumor growth inhibition in mice models, suggesting cynarin’s direct antitumor potential. Notably, the use of UHPLC-QqQ-MS/MS in this study strengthens the credibility of these findings by providing an exact method for identifying active compounds. Jafari et al (35) explored cynarin’s cytotoxic effects on gastric adenocarcinoma (AGS) cells and found that cynarin, among other phenolic compounds, induced cell cycle arrest and apoptosis. The observed morphological changes and the influence on critical apoptosis and cell cycle regulators, including the upregulation of Bax and caspase-3 and the downregulation of cyclin D1 and Bcl2, further support cynarin’s pro-apoptotic properties. Lastly, Angelini et al (36) investigated the role of cynarin in overcoming multidrug resistance in human uterine sarcoma cells. Their findings revealed that cynarin can increase the intracellular accumulation and cytotoxicity of doxorubicin. This effect is attributed to cynarin’s ability to inhibit P-glycoprotein-mediated transport, a common mechanism underlying multidrug resistance in cancer cells. These results imply that cynarin could act as an adjunctive agent in chemotherapy, potentially enhancing the efficacy of conventional anticancer drugs. The results of the present study demonstrated that cynarin forms three hydrogen bonds (H-bonds) with specific amino acid residues (Phe161, Glu234, and Asp274) within the active site of Akt1. This information provides valuable insights into the potential molecular interactions between cynarin and Akt1, which may be crucial for understanding its biological effects.
Rosmarinic acid, a polyphenol with diverse bioactivities, exhibits antiviral, antibacterial, anti-inflammatory, and antioxidant properties. Additionally, it represents promising effects on diabetes, cancer, neuroprotection, and liver protection (37,38). This naturally occurring compound is abundantly present in plants of the Lamiaceae family and is biosynthesized from the amino acids L-phenylalanine and L-tyrosine (37). Interestingly, research suggests that plant cell cultures can accumulate rosmarinic acid at concentrations exceeding those found in the whole plant, hinting at the potential for biotechnological production (37). The pharmacokinetics of rosmarinic acid have been investigated in both animals and humans. Studies reveal its versatility in administration routes, including topical, pulmonary, intranasal, and intravenous delivery. However, oral ingestion remains the primary route of absorption in humans (39). This CAD has been linked to various health benefits, including improvements in skin conditions, allergies, osteoarthritis, cognitive function, and metabolic syndrome management. Notably, its use is generally well-tolerated, with no serious adverse effects (39).
The multifarious anticancer attributes of rosmarinic acid have been demonstrated across diverse cancer cell lines, including ovarian (40), oral (41), hepatic (41), gastric (42), and an array of cell lines evaluated for the efficacy of silver nanoparticles (43). The mechanisms through which rosmarinic acid exerts its antineoplastic effects are diverse, implicating pathways such as apoptosis induction (40-42,44), cell cycle arrest (41), suppression of cellular migration and invasion (40,43,44), and modulation of cancer-related gene expression (40,42). The induction of programmed cell death was a recurrent theme across the studies, with evidence of chromatin condensation, DNA fragmentation (40), and activation of caspases (42,44). This apoptotic response appears to be mediated through intrinsic and extrinsic pathways, as indicated by increased caspase-9 mRNA expression (42). Luo et al (41) highlighted the role of rosmarinic acid in cell cycle modulation, particularly at the G2/M checkpoint (41) in human oral cancer cells, which is critical for preventing the proliferation of damaged cells. Another significant finding across these studies is the effect of rosmarinic acid on cellular migration and invasion, which are essential steps of cancer metastasis. Rosmarinic acid substantially inhibited these processes (40,43,44), suggesting its potential utility in preventing cancer dissemination. Moreover, the modulation of long non-coding RNA MALAT-1 expression (40) and the impact on endoplasmic reticulum stress (41) further represent the intricate interactions of rosmarinic acid with cellular pathways to exert its antineoplastic effects. The synthesis of silver nanoparticles using a rosmarinic acid extract by Netala et al (43) introduced an innovative approach to enhancing the bioavailability and therapeutic potential of rosmarinic acid. According to the present results, rosmarinic acid formed six hydrogen and four hydrophobic interactions with Leu156, Gly157, Val164, Ala177, Glu234, Glu278, and Met281 inside the Akt1 catalytic cleft.
The current study offers valuable preliminary evidence regarding the inhibitory potential of CADs against Akt1. However, several essential considerations warrant further investigation. Firstly, the in silico nature of the computational molecular docking simulations employed here necessitates experimental validation through in vitro and in vivo models. This is critical to corroborate the observed binding affinities and anticancer effects within a more comprehensive biological context. Secondly, exploring the possible synergistic effects of these compounds with established cancer therapies and their efficacy in overcoming drug resistance would be valuable in fully elucidating their potential clinical utility.
Conclusion
In general, this in silico investigation identified cynarin and rosmarinic acid, two naturally occurring CADs, as potential Akt1 inhibitors. The molecular docking simulations suggest these compounds exhibit superior binding affinity to Akt1 compared to capivasertib, a well-established Akt1 inhibitor. This finding warrants further exploration of their therapeutic potential in targeting the PI3K/Akt pathway, which is dysregulated in various malignancies such as OSCC. As previously documented in research, the anticancer properties of these natural compounds provide additional support for their potential. These prior studies have shown promise in areas including apoptosis induction, cellular proliferation suppression, and metastasis inhibition.
Acknowledgments
The authors acknowledge the support of the Deputy of Research and Technology, Research Center for Molecular Medicine, and Dental Research Center, Hamadan University of Medical Sciences, Hamadan, Iran.
Author’s Contribution
Conceptualization: Amir Taherkhani, Shokoofeh Jamshidi, and Setareh Shojaei.
Data curation: Amir Taherkhani, Amirhoseyn Naiini, and Setareh Shojaei.
Formal analysis: Amir Taherkhani, Amirhoseyn Naiini, and Setareh Shojaei.
Investigation: Amir Taherkhani and Shokoofeh Jamshidi.
Methodology: Amir Taherkhani.
Project administration: Amir Taherkhani.
Resources: Amir Taherkhani.
Software: Amir Taherkhani, Amirhoseyn Naiini, and Setareh Shojaei.
Supervision: Amir Taherkhani and Setareh Shojaei.
Validation: Amir Taherkhani and Setareh Shojaei.
Visualization: Amir Taherkhani.
Writing–original draft: Amir Taherkhani.
Writing–review & editing: Setareh Shojaei and Shokoofeh Jamshidi.
Competing Interests
The authors declare that they have no competing interests.
Consent for Publication
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the currentstudy are available from the corresponding author upon reasonable request.
Ethical Approval
The present study was confirmed by the Ethics Committee of Hamadan University of Medical Sciences, Hamadan, Iran (IR.UMSHA.REC.1401.902).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Nakashiro K, Tanaka H, Goda H, Iwamoto K, Tokuzen N, Hara S. Identification of Akt1 as a potent therapeutic target for oral squamous cell carcinoma. Int J Oncol 2015; 47(4):1273-81. doi: 10.3892/ijo.2015.3134 [Crossref] [ Google Scholar]
- Huang H, Yi JK, Lim SG, Park S, Zhang H, Kim E. Costunolide induces apoptosis via the reactive oxygen species and protein kinase B pathway in oral cancer cells. Int J Mol Sci 2021; 22(14):7509. doi: 10.3390/ijms22147509 [Crossref] [ Google Scholar]
- Islam M, Jones S, Ellis I. Role of AKT/protein kinase B in cancer metastasis. Biomedicines 2023; 11(11):3001. doi: 10.3390/biomedicines11113001 [Crossref] [ Google Scholar]
- Sun EC, Dong SS, Li ZJ, Li CX. Clinicopathological significance of AKT1 and PLK1 expression in oral squamous cell carcinoma. Dis Markers 2022; 2022:7300593. doi: 10.1155/2022/7300593 [Crossref] [ Google Scholar]
- Che Y, Zhang H, Li H, Wu X. CIP2A interacts with AKT1 to promote the malignant biological behaviors of oral squamous cell carcinoma by upregulating the GSK-3β/β-catenin pathway. Exp Ther Med 2023; 26(5):514. doi: 10.3892/etm.2023.12213 [Crossref] [ Google Scholar]
- Zhao L, Lu X, Cao Y. MicroRNA and signal transduction pathways in tumor radiation response. Cell Signal 2013; 25(7):1625-34. doi: 10.1016/j.cellsig.2013.04.004 [Crossref] [ Google Scholar]
- Manoochehri Khoshinani H, Afshar S, Sedighi Pashaki A, Mahdavinezhad A, Nikzad S, Najafi R. Involvement of miR-155/FOXO3a and miR-222/PTEN in acquired radioresistance of colorectal cancer cell line. Jpn J Radiol 2017; 35(11):664-72. doi: 10.1007/s11604-017-0679-y [Crossref] [ Google Scholar]
- Li XN, Hua LX, Zhou TS, Wang KB, Wu YY, Emam M. Cinnamic acid derivatives: inhibitory activity against Escherichia coli β-glucuronidase and structure-activity relationships. J Enzyme Inhib Med Chem 2020; 35(1):1372-8. doi: 10.1080/14756366.2020.1780225 [Crossref] [ Google Scholar]
- Aba PE, Ihedioha JI, Asuzu IU. A review of the mechanisms of anti-cancer activities of some medicinal plants-biochemical perspectives. J Basic Clin Physiol Pharmacol 2023; 34(4):419-28. doi: 10.1515/jbcpp-2021-0257 [Crossref] [ Google Scholar]
- Albahri G, Badran A, Abdel Baki Z, Alame M, Hijazi A, Daou A. Potential anti-tumorigenic properties of diverse medicinal plants against the majority of common types of cancer. Pharmaceuticals (Basel) 2024; 17(5):574. doi: 10.3390/ph17050574 [Crossref] [ Google Scholar]
- Liu P, Ying J, Guo X, Tang X, Zou W, Wang T. An exploration of the effect of Chinese herbal compound on the occurrence and development of large intestine cancer and intestinal flora. Heliyon 2024; 10(1):e23533. doi: 10.1016/j.heliyon.2023.e23533 [Crossref] [ Google Scholar]
- Memarzia A, Saadat S, Asgharzadeh F, Behrouz S, Folkerts G, Boskabady MH. Therapeutic effects of medicinal plants and their constituents on lung cancer, in vitro, in vivo and clinical evidence. J Cell Mol Med 2023; 27(19):2841-63. doi: 10.1111/jcmm.17936 [Crossref] [ Google Scholar]
- Olaleye OO, Kim DH, Spriggs KA. Antiproliferative activities of some selected Nigerian medicinal plants against breast, liver, and cervical cancer cells. BMC Complement Med Ther 2024; 24(1):110. doi: 10.1186/s12906-024-04365-w [Crossref] [ Google Scholar]
- Rani DM, Wongso H, Purwoko RY, Winarto NB, Shalas AF, Triatmoko B. Anti-cancer bioprospecting on medicinal plants from Indonesia: a review. Phytochemistry 2023; 216:113881. doi: 10.1016/j.phytochem.2023.113881 [Crossref] [ Google Scholar]
- Singla RK, Wang X, Gundamaraju R, Joon S, Tsagkaris C, Behzad S. Natural products derived from medicinal plants and microbes might act as a game-changer in breast cancer: a comprehensive review of preclinical and clinical studies. Crit Rev Food Sci Nutr 2023; 63(33):11880-924. doi: 10.1080/10408398.2022.2097196 [Crossref] [ Google Scholar]
- Chandra S, Roy A, Jana M, Pahan K. Cinnamic acid activates PPARα to stimulate lysosomal biogenesis and lower amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol Dis 2019; 124:379-95. doi: 10.1016/j.nbd.2018.12.007 [Crossref] [ Google Scholar]
- Ruwizhi N, Aderibigbe BA. Cinnamic acid derivatives and their biological efficacy. Int J Mol Sci 2020; 21(16):5712. doi: 10.3390/ijms21165712 [Crossref] [ Google Scholar]
- Abd El-Raouf OM, El-Sayed EM, Manie MF. Cinnamic acid and cinnamaldehyde ameliorate cisplatin-induced splenotoxicity in rats. J Biochem Mol Toxicol 2015; 29(9):426-31. doi: 10.1002/jbt.21715 [Crossref] [ Google Scholar]
- Wang R, Yang W, Fan Y, Dehaen W, Li Y, Li H. Design and synthesis of the novel oleanolic acid-cinnamic acid ester derivatives and glycyrrhetinic acid-cinnamic acid ester derivatives with cytotoxic properties. Bioorg Chem 2019; 88:102951. doi: 10.1016/j.bioorg.2019.102951 [Crossref] [ Google Scholar]
- Guo S, Zhen Y, Zhu Z, Zhou G, Zheng X. Cinnamic acid rescues behavioral deficits in a mouse model of traumatic brain injury by targeting miR-455-3p/HDAC2. Life Sci 2019; 235:116819. doi: 10.1016/j.lfs.2019.116819 [Crossref] [ Google Scholar]
- Koczurkiewicz-Adamczyk P, Piska K, Gunia-Krzyżak A, Bucki A, Jamrozik M, Lorenc E. Cinnamic acid derivatives as chemosensitising agents against DOX-treated lung cancer cells - involvement of carbonyl reductase 1. Eur J Pharm Sci 2020; 154:105511. doi: 10.1016/j.ejps.2020.105511 [Crossref] [ Google Scholar]
- Martínez-Rosas JR, Díaz-Torres R, Ramírez-Noguera P, López-Barrera LD, Escobar-Chavez JJ, Ángeles ER. PLGA nanoparticles of a new cinnamic acid derivative inhibits cellular proliferation on breast cancer cell line MCF-7 in a PPARγ dependent way. Pharmazie 2020; 75(7):324-8. doi: 10.1691/ph.2020.0450 [Crossref] [ Google Scholar]
- Imai M, Yokoe H, Tsubuki M, Takahashi N. Growth inhibition of human breast and prostate cancer cells by cinnamic acid derivatives and their mechanism of action. Biol Pharm Bull 2019; 42(7):1134-9. doi: 10.1248/bpb.b18-01002 [Crossref] [ Google Scholar]
- Anantharaju PG, Reddy DB, Padukudru MA, Kumari Chitturi CM, Vimalambike MG, Madhunapantula SV. Induction of colon and cervical cancer cell death by cinnamic acid derivatives is mediated through the inhibition of histone deacetylases (HDAC). PLoS One 2017; 12(11):e0186208. doi: 10.1371/journal.pone.0186208 [Crossref] [ Google Scholar]
- de Freitas Meirelles LE, de Souza MV, Carobeli LR, Morelli F, Mari NL, Damke E. Combination of conventional drugs with biocompounds derived from cinnamic acid: a promising option for breast cancer therapy. Biomedicines 2023; 11(2):275. doi: 10.3390/biomedicines11020275 [Crossref] [ Google Scholar]
- Issa NT, Badiavas EV, Schürer S. Research techniques made simple: molecular docking in dermatology - a foray into in silico drug discovery. J Invest Dermatol 2019;139(12):2400-8.e1. 10.1016/j.jid.2019.06.129.
- Wanigasooriya K, Tyler R, Barros-Silva JD, Sinha Y, Ismail T, Beggs AD. Radiosensitising cancer using phosphatidylinositol-3-kinase (PI3K), protein kinase B (AKT) or mammalian target of rapamycin (mTOR) inhibitors. Cancers (Basel) 2020; 12(5):1278. doi: 10.3390/cancers12051278 [Crossref] [ Google Scholar]
- Del Mar Maldonado M, Medina JI, Velazquez L, Dharmawardhane S. Targeting Rac and Cdc42 GEFs in metastatic cancer. Front Cell Dev Biol 2020; 8:201. doi: 10.3389/fcell.2020.00201 [Crossref] [ Google Scholar]
- Xiong S, Su X, Kang Y, Si J, Wang L, Li X. Effect and mechanism of chlorogenic acid on cognitive dysfunction in mice by lipopolysaccharide-induced neuroinflammation. Front Immunol 2023; 14:1178188. doi: 10.3389/fimmu.2023.1178188 [Crossref] [ Google Scholar]
- Ramorobi LM, Matowane GR, Mashele SS, Bonnet SL, Noreljaleel AE, Swain SS. Bioactive synergism between zinc mineral and p-coumaric acid: a multi-mode glycemic control and antioxidative study. J Food Biochem 2022; 46(10):e14360. doi: 10.1111/jfbc.14360 [Crossref] [ Google Scholar]
- Yarmohammadi E, Khanjani M, Khamverdi Z, Savari M, Taherkhani A. Herbal metabolites as potential carbonic anhydrase inhibitors: promising compounds for cancer and metabolic disorders. J Obes Metab Syndr 2023; 32(3):247-58. doi: 10.7570/jomes23029 [Crossref] [ Google Scholar]
- Bayat Z, Tarokhian A, Taherkhani A. Cinnamic acids as promising bioactive compounds for cancer therapy by targeting MAPK3: a computational simulation study. J Complement Integr Med 2023; 20(3):621-30. doi: 10.1515/jcim-2023-0046 [Crossref] [ Google Scholar]
- Malekipour MH, Shirani F, Moradi S, Taherkhani A. Cinnamic acid derivatives as potential matrix metalloproteinase-9 inhibitors: molecular docking and dynamics simulations. Genomics Inform 2023; 21(1):e9. doi: 10.5808/gi.22077 [Crossref] [ Google Scholar]
- Zhang M, Wang YW, Zhu YZ, Gu XL. Discovery of quality control ingredients in burdock root by combining anti-tumor effects and UHPLC-QqQ-MS/MS. Biomed Chromatogr 2021; 35(11):e5187. doi: 10.1002/bmc.5187 [Crossref] [ Google Scholar]
- Jafari N, Zargar SJ, Delnavazi MR, Yassa N. Cell cycle arrest and apoptosis induction of phloroacetophenone glycosides and caffeoylquinic acid derivatives in gastric adenocarcinoma (AGS) cells. Anticancer Agents Med Chem 2018; 18(4):610-6. doi: 10.2174/1871520618666171219121449 [Crossref] [ Google Scholar]
- Angelini A, Di Pietro R, Centurione L, Castellani ML, Conti P, Porreca E. Inhibition of P-glycoprotein-mediated transport by S-adenosylmethionine and cynarin in multidrug-resistant human uterine sarcoma MES-SA/Dx5 cells. J Biol Regul Homeost Agents 2012; 26(3):495-504. [ Google Scholar]
- Petersen M, Simmonds MS. Rosmarinic acid. Phytochemistry 2003; 62(2):121-5. doi: 10.1016/s0031-9422(02)00513-7 [Crossref] [ Google Scholar]
- Guan H, Luo W, Bao B, Cao Y, Cheng F, Yu S. A comprehensive review of rosmarinic acid: from phytochemistry to pharmacology and its new insight. Molecules 2022; 27(10):3292. doi: 10.3390/molecules27103292 [Crossref] [ Google Scholar]
- Hitl M, Kladar N, Gavarić N, Božin B. Rosmarinic acid-human pharmacokinetics and health benefits. Planta Med 2021; 87(4):273-82. doi: 10.1055/a-1301-8648 [Crossref] [ Google Scholar]
- Zhang Y, Hu M, Liu L, Cheng XL, Cai J, Zhou J. Anticancer effects of rosmarinic acid in OVCAR-3 ovarian cancer cells are mediated via induction of apoptosis, suppression of cell migration and modulation of lncRNA MALAT-1 expression. J BUON 2018; 23(3):763-8. [ Google Scholar]
- Luo Y, Ma Z, Xu X, Qi H, Cheng Z, Chen L. Anticancer effects of rosmarinic acid in human oral cancer cells is mediated via endoplasmic reticulum stress, apoptosis, G2/M cell cycle arrest and inhibition of cell migration. J BUON 2020; 25(2):1245-50. [ Google Scholar]
- Radziejewska I, Supruniuk K, Bielawska A. Anti-cancer effect of combined action of anti-MUC1 and rosmarinic acid in AGS gastric cancer cells. Eur J Pharmacol 2021; 902:174119. doi: 10.1016/j.ejphar.2021.174119 [Crossref] [ Google Scholar]
- Netala VR, Hou T, Sana SS, Li H, Zhang Z. Rosmarinic acid-rich Perilla frutescens extract-derived silver nanoparticles: a green synthesis approach for multifunctional biomedical applications including antibacterial, antioxidant, and anticancer activities. Molecules 2024; 29(6):1250. doi: 10.3390/molecules29061250 [Crossref] [ Google Scholar]
- Chen X, Su HZZ. Detailed studies on the anticancer action of rosmarinic acid in human Hep-G2 liver carcinoma cells: evaluating its effects on cellular apoptosis, caspase activation and suppression of cell migration and invasion. J BUON 2020; 25(4):2011-6. [ Google Scholar]