Avicenna J Dent Res. 16(2):90-96.
doi: 10.34172/ajdr.1844
Original Article
Antimicrobial Efficacy of Carbonated Hydroxyapatite Against Streptococcus mutans, Enterococcus faecalis, and Candida albicans: An In Vitro Study
S.Swathi Priyadharshini 1, 2  , Chinnasamy Ragavendran 1, *, Anand Sherwood 2, Rathna Piriyanga 2
, Chinnasamy Ragavendran 1, *, Anand Sherwood 2, Rathna Piriyanga 2
Author information:
1Department of Cariology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Science, (SIMATS), saveetha university, Chennai, Tamil Nadu 600077, India
2Department of Conservative Dentistry and Endodontics, C.S.I. College of Dental Sciences and Research, Madurai, Tamil Nadu 625001, India
Abstract
Background: Dental caries commonly lead to pulp infection, necessitating vital pulp therapy (VPT) to protect the health of the pulp tissue. Effective pulp-capping materials, including those with antimicrobial properties, are crucial for successful VPT. This study aimed to evaluate the antimicrobial efficacy of carbonated hydroxyapatite (CHA), a novel pulp-capping material, compared to frequently used materials, against Streptococcus mutans, Enterococcus faecalis, and Candida albicans.
Methods: CHA was synthesized at three concentrations (0.05 M, 0.1 M, and 0.5 M) and compared with mineral trioxide aggregate (MTA). Antimicrobial efficacy was evaluated using the agar diffusion method. Zones of inhibition were evaluated after 24 hours of incubation. A one-way analysis of variance (ANOVA) and post hoc tests were used for the statistical analysis.
Results: All materials exhibited antimicrobial activity against the tested microorganisms, with CHA at 0.1 M concentration demonstrating the highest efficacy against E. faecalis and C. albicans. MTA and CHA at 0.5 M concentrations showed strong activity against S. mutans. According to statistical analysis, significant differences were found in antibacterial activity among the tested materials (P<0.0001).
Conclusion: CHA, particularly at 0.1 M concentration, demonstrated significant antimicrobial efficacy against S. mutans, E. faecalis, and C. albicans, suggesting its potential as a pulp-capping agent. Further research is warranted to explore its clinical applicability in VPT.
Keywords: Anti-bacterial, Biomaterials, Enterococcus faecalis, Pulp capping agents, Streptococcus mutans
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Priyadharshini SS, Ragavendran C, Anand Sherwood A, Piriyanga R. Antimicrobial efficacy of carbonated hydroxyapatite against Streptococcus mutans, Enterococcus faecalis, and Candida albicans: an in vitro study. Avicenna J Dent Res. 2024; 16(2):90-96. doi:10.34172/ajdr.1844
Background
Dental caries causes the degradation of mineralized tooth structure and represents the predominant cause of pulp infection (1). Vital pulp therapy (VPT) endeavours to preserve the viability and functionality of dental pulp tissue that has sustained injury. Such injuries can be attributed to various aetiologies, encompassing carious lesions, operative interventions, iatrogenic mishaps, or traumatic dental events (2). The effectiveness of materials used for pulp capping plays a critical role in determining the outcome of endodontic procedures.
The use of antimicrobial pulp-capping materials under permanent restorations has become essential to protect the dental pulp (3). To remove any remaining bacteria in dentin that has decayed, to encourage the regeneration of lost tissue, and to stop subsequent infections and the risk of pulp injury, a material used in pulp capping procedures should ideally have sufficient mechanical, physicochemical, and antibacterial properties (4).
Despite numerous studies assessing the antimicrobial properties of various pulp-capping materials, there remains a lack of consensus regarding using carbonated hydroxyapatite (CHA) for this application. CHA is a variant of hydroxyapatite (HA) that has been investigated for its potential in various biomedical applications, including its antibacterial properties. The incorporation of carbonate ions into the HA structure is thought to enhance its biological properties, making it more similar to the mineral of human bones (5). Studies have shown that silver-loaded coralline HA possesses promising antibacterial activity against E. coli and Staphylococcus aureus, attributed to the presence of Ag + ions (6). Similarly, CHA combined with propolis, a natural antibacterial agent, has exhibited effective antibacterial activity against Aggregatibacter actinomycetemcomitans (7). Compared to existing research, these findings highlight the importance of doping or combining HA with antibacterial agents to enhance its antibacterial activity. However, there is a lack of research comparing the antimicrobial efficacy of undoped CHA as a novel pulp capping material to traditional materials.
Streptococcus mutans comprises a cluster of seven bacterial species closely associated with the pathogenesis of caries in humans, with S. mutans recognized as the primary etiological factor in this condition (8). E. faecalis strains are obtained from the saliva, pulp chamber, and root canals of patients undergoing endodontic procedures (9). The presence of these bacteria in the root canals may be due to their migration from the oral cavity during or after endodontic treatment or through cavities (10). C. albicans metabolizes glucose and maltose, generating both acid and gas. It exhibits notable acidogenic capabilities and has demonstrated a propensity for biofilm formation (11).
Midway through the 1990s, mineral trioxide aggregate (MTA)—a cement based on calcium silicate with superior biological and physical qualities—was brought to the field of dentistry (12). MTA has been found to be a biocompatible dental material in several investigations. Its biological qualities may be attributed to its high alkalinity, induction of hard tissue development, superior sealing ability, and antibacterial activities (13). Considering MTA’s physical and chemical properties, it has been proposed that it can be employed as a biomaterial for various endodontic treatments (14).
Non-stoichiometric, nanoscale CHA, characterized by low crystallinity and containing carbonate ions, presents itself as a promising alternative for various applications, treatments, and therapies associated with bone-related conditions (15). Due to its high osteoconductive qualities, good biodegradation in the body, and organic matter similar to that of bone, CHA is perfect as a material for bone grafting (16). However, the efficacy of CHA as a pulp capping agent remains unassessed at present.
This study seeks to fill the knowledge gap by comparing the antimicrobial efficacy of a novel pulp capping material to other commonly used materials. The research question is, what is the antimicrobial efficacy of CHA, a novel pulp capping material, compared to other frequently used materials?
This research aims to compare the antimicrobial efficacy of CHA, a novel pulp capping material, to other commonly utilized materials against S. mutans, E. faecalis, and C. albicans. The hypothesis to be examined is that “the novel pulp capping material will not exhibit superior antimicrobial efficacy in comparison to other commonly used materials.”
Materials and Methods
The antibacterial effectiveness of CHA against S. mutans, E. faecalis, and C. albicans was evaluated using the agar diffusion method. The bacterial strains S. mutans MTCC-860 and E. faecalis MTCC439 were cultured in brain-heart infusion broth under anaerobic conditions at 37 °C. C. albicans strain MTCC-183 was cultured on Sabouraud dextrose agar plates and maintained at a temperature of 37°C.
Preparation of Carbonated Hydroxyapatite
The synthesis of CHA at various concentrations using the precipitation technique was conducted according to the methodology outlined by Othman et al (17). Three distinct concentrations (i.e., 0.05 M, 0.1 M, and 0.5 M) were prepared, and the synthesized material was characterized through X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM) analysis.
X-ray Diffraction Analysis
XRD analysis of the samples synthesized with three different concentrations (0.05 M, 0.1 M, and 0.5 M) revealed characteristic diffraction peaks at 25.8°, 28.1°, 29.0°, 31.7°, 32.2°, 32.8°, 34.0°, 39.7°, 46.6°, 48.1°, 49.4°, 53.1°, and 55.9°. These peaks correspond to the crystal lattice reflections of HA, according to the International Centre for Diffraction Data (ICDD 09-0432), confirming the presence of CHA in the samples.
Fourier-Transform Infrared Spectroscopy Analysis
Figure 1 displays the FTIR spectrum of the CHA sample. This spectrum exhibits characteristic absorption bands corresponding to various functional groups present within the material. Notably, the spectrum includes several key bands typically observed in CHA spectra, which are as follows:
Carbonate Vibrations: Bands in the region 1350–1550 cm⁻¹ correspond to carbonate (CO3) vibrations, indicating the substitution of carbonate ions into the phosphate lattice of the HA.
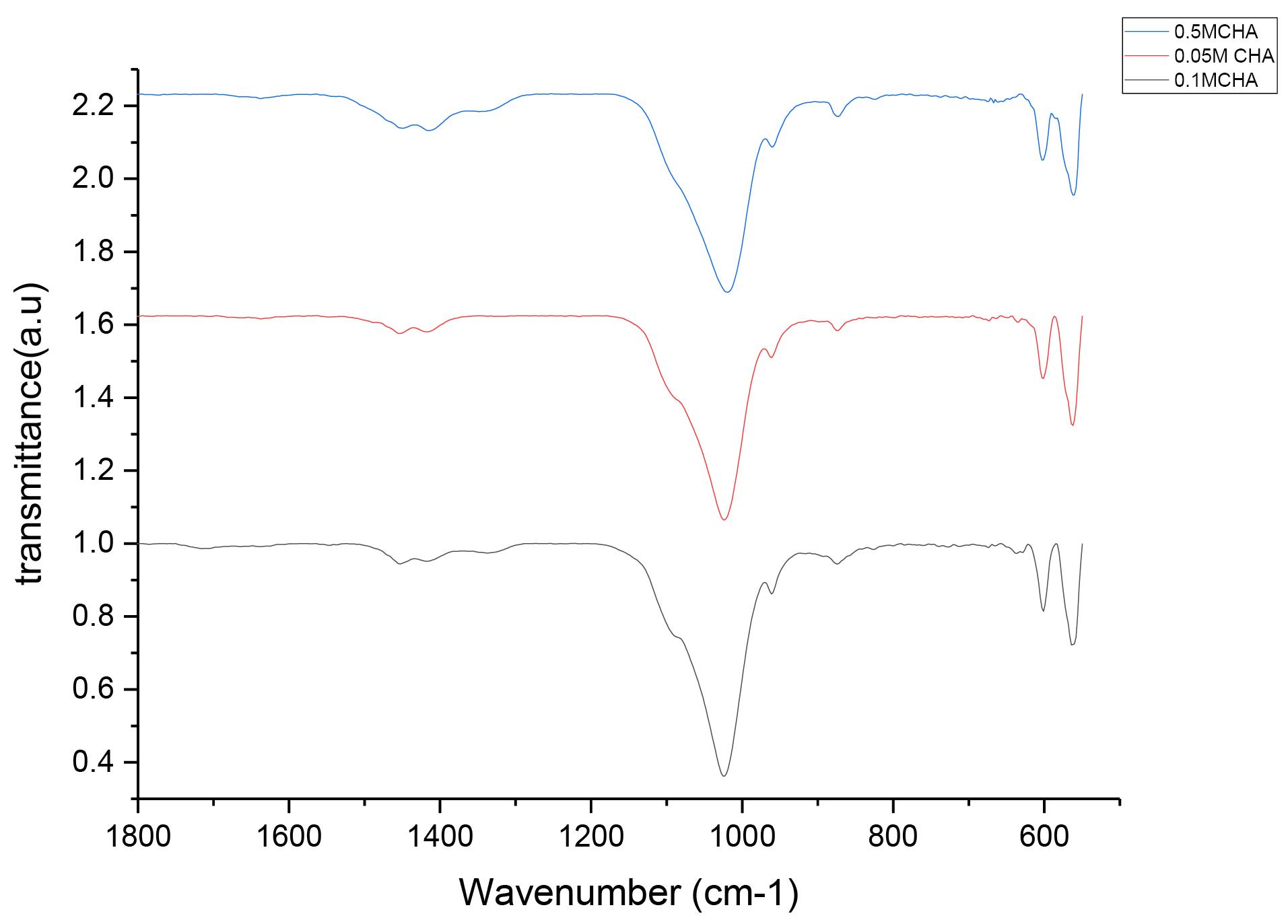
Figure 1.
FTIR Spectra of Different Concentrations of CHA. Note. FTIR: Fourier-transform infrared spectroscopy; CHA: Carbonated hydroxyapatite
.
FTIR Spectra of Different Concentrations of CHA. Note. FTIR: Fourier-transform infrared spectroscopy; CHA: Carbonated hydroxyapatite
Phosphate Vibrations: Bands in the regions 1022 cm⁻¹ and 1090 cm⁻¹ correspond to phosphate (PO4) vibrations, demonstrating the presence of the phosphate group in HA.
Hydroxyl Vibrations: Bands around 630–670 cm⁻¹ correspond to hydroxyl (OH) vibrations, implying the characteristic of HA.
Scanning Electron Microscopy Analysis
Figure 2 depicts SEM images [(a), (b), and (c)] of the CHA samples, providing a characterization of the material’s surface morphology. The materials exhibited plate-like surface morphology. Following the characterization process, the antimicrobial effectiveness of the material was assessed against three distinct microorganisms, including S. mutans, E. faecalis, and C. albicans.

Figure 2.
Scanning electron microscopy micrographs of different concentrations of CHA. (a) 0.05 M, (b) 0.1 M, and (c) 0.5 M
.
Scanning electron microscopy micrographs of different concentrations of CHA. (a) 0.05 M, (b) 0.1 M, and (c) 0.5 M
Mineral Trioxide Aggregate (Biostructure Mineral Trioxide Aggregate, Safe Endo, Gujarat, India)
This is available in powder and liquid form.The composition of powder includes tricalcium silicate, dicalcium silicate, zirconium oxide, tricalcium aluminate, and tetracalcium aluminoferrite The liquid consists of calcium chloride. Powder was incorporated into the liquid following the guidelines provided by the manufacturer and was later filled into its respective pits.
The Agar Diffusion Method for the Assessment of Antimicrobial Activity
The agar disc diffusion test was conducted following the methodology described in previous research (18). The microorganisms were then inoculated into sterile test tubes containing 1.5 mL of sterile broth cultures, such as brain-heart infusion for E. faecalis, blood agar for S. mutans, and Sabouraud Dextrose Agar for C. albicans. The overnight-grown broth cultures were diluted to match the 0.5 McFarland turbidity standard before inoculation. Spread plates were then created by inoculating 100 mL of each bacterial culture (1.5 × 108 cells) on Mueller-Hinton agar. To assess the antibacterial activities of freshly prepared pastes, different concentrations of CHA and MTA were used to fill designated wells (5 mm in diameter) created within agar plates previously inoculated with the respective test bacteria.
After adding the pulp capping materials to the medium, it was pre-incubated for an hour at room temperature and then incubated for a full day at 37 °C. Chloramphenicol (10 µg/mL) was utilized as a positive control. Next, the zone of inhibition of the samples for the target bacterial strain was visualized by measuring the diameter of the inhibition zone around the well using a ruler with a precision of 0.5 mm. The diameter of the inhibition zones was determined using the following formula:
Size of inhibition zone = (I – D) / 2
where I and D represent the mean value of three independent measurements of the inhibition halo diameter (mm) and the diameter of the well created in the agar plate (5 mm), respectively. This experiment was performed in triplicate for each bacterial strain.
Statistical Analysis
Data collection involved preparing agar plates with the test agents and measuring the zone of inhibition.Data analysis encompassed calculating the mean and standard deviation of the zone of inhibition for each test agent. SPSS (version 20.0) was used for the statistical analysis (SPSS Inc., Chicago, IL, USA). A one-way analysis of variance (ANOVA) was employed in the statistical analysis to compare the mean zone of inhibition for each test substance.
Results
All the materials produced inhibition zones of 7–10 mm against S. mutans (Figure 3a,b,c), 8–11 mm against E. faecalis, and 7–12 mm against C. albicans. The results revealed that CHA at a concentration of 0.1 M had the highest antimicrobial activity against E. faecalis and C. albicans. The inhibition zones of the four materials were significantly smaller than those of NaOCl.Table 1 presents the average zone of inhibition of S. mutans observed with the tested materials at 24 hours. Based on the results, 0.5 M carbonated hydroxyapatite (CHA) and MTA exhibited the greatest zone of inhibition at 9.6 ± 0.57 mm, followed by 0.05 M CHAP at 8.6 ± 0.57 mm and 0.1 M CHAP at 7.3 ± 0.57 mm.
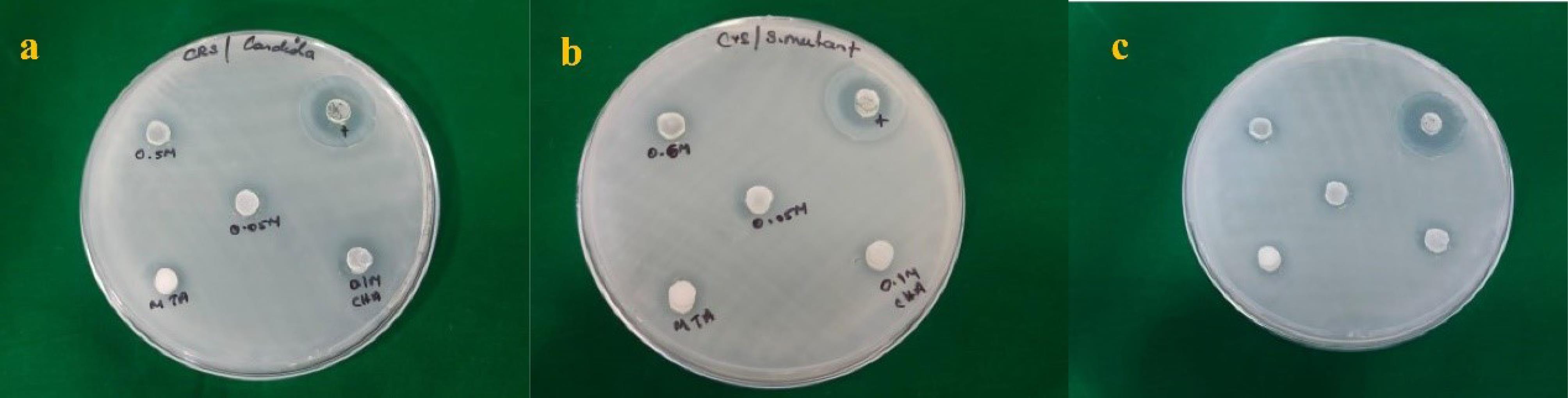
Figure 3.
Zone of Inhibition for Different Concentrations of CHAP Against (a) Candida albicans, (b) Streptococcus mutans, and (c) Enterococcus faecalis. Note. CHA: Carbonated Hydroxyapatite
.
Zone of Inhibition for Different Concentrations of CHAP Against (a) Candida albicans, (b) Streptococcus mutans, and (c) Enterococcus faecalis. Note. CHA: Carbonated Hydroxyapatite
Table 1.
Mean Zone of Inhibition of Streptococcus mutans With Tested Materials at 24 Hours
|
Tested Material
|
S.
mutans
Mean (mm)+SD
|
| 0.05 CHA |
8.6 + 0.57 |
| 0.1 CHA |
7.3 + 0.57 |
| 0.5 CHA |
9.6 + 0.57 |
| MTA |
9.6 + 0.57 |
Note. SD: Standard deviation; CHA: Carbonated Hydroxyapatite; MTA: Mineral trioxide aggregate.
Table 2 provides the average zone of inhibition of E. faecalis observed with tested materials at 24 hours. The findings demonstrated that 0.1 M CHAP and MTA had the highest zone of inhibition at 10.6 ± 0.57 mm, followed by 0.5 M CHAP at 8.6 ± 0.57 mm and 0.05 M CHAP at 7.6 ± 0.57 mm.
Table 2.
Mean Zone of Inhibition Enterococcus faecalis With Tested Materials at 24 Hours
|
Tested Material
|
E. faecalis
Mean (mm)+SD
|
| 0.05 CHA |
7.6 + 0.57 |
| 0.1 CHA |
10.6 + 0.57 |
| 0.5 CHA |
8.6 + 0.57 |
| MTA |
10.6 + 0.57 |
Note. SD: Standard deviation; CHA: Carbonated Hydroxyapatite; MTA: Mineral trioxide aggregate.
Table 3 summarizes the data on the average zone of inhibition of C. albicans detected with tested materials after 24 hours. According to the obtained data, 0.1 M CHAP had the greatest zone of inhibition at 11.6 ± 0.57 mm, followed by MTA at 8.6 ± 0.57 mm, 0.5 M CHAP at 7.6 ± 0.5 mm, and 0.05 M CHAP at 7.0 ± 0.5 mm.An intergroup comparison was conducted to assess the antimicrobial activity and variations within the tested materials, employing ANOVA as the statistical method. The findings showed that there was a statistically significant difference (P < 0.0001) between the groups.
Table 3.
Mean Zone of Inhibition Candida albicans With Tested Materials at 24 Hours
|
Tested Material
|
C. albicans
Mean (mm)+SD
|
| 0.05 CHA |
7.0 + 0.57 |
| 0.1 CHA |
11.6 + 0.57 |
| 0.5 CHA |
7.6 + 0.57 |
| MTA |
8.6 + 0.57 |
Note. SD: Standard deviation; CHA: Carbonated Hydroxyapatite MTA: Mineral trioxide aggregate.
Discussion
This research sought to examine the antimicrobial effects of CHA at three different concentrations and compare them with those of other pulp capping agents using the agar diffusion test. Our findings indicated that CHA at a concentration of 0.1 M represented significant antimicrobial activity against C. albicans and E. faecalis, while 0.5 M demonstrated significant antimicrobial activity against S. mutans. Previous studies have reported the potential use of CHA as a biomaterial in dental applications, but its antimicrobial properties have not been extensively studied yet. Understanding the antimicrobial properties of CHA is essential for its future use as a pulp-capping agent in endodontic therapy.
In this study, the antimicrobial properties of CHA were compared with those of the commonly used pulp capping agent (MTA). The rationale for choosing S. mutans was that it is a significant contributor to dental caries and, therefore, a suitable microorganism for testing a material’s ability to suppress cariogenic bacteria. Moreover, S. mutansis widely recognized as a primary pathogen in the production of oral biofilms. E. faecalis was selected for susceptibility testing due to its involvement in endodontic failures and primary root canal infections, establishing its clinical relevance in assessing antimicrobial efficacy (19). Fungi, such as C. albicans, might enter the root canal from the oral cavity due to coronal leakage or inadequate asepsis during endodontic therapy (20). The agar diffusion test is a well-established method for evaluating the antibacterial activity of dental materials, having been extensively employed and verified in antimicrobial research.
Nanostructured CHA was employed for alveolar bone repair in a randomized clinical study conducted by Resende et al. Ninety days after surgery, the samples showed a statistically significant increase (P < 0.05) in new bone formation in the CHA group when compared with Bio-Oss® (21). When it comes to bone repairs, the dental profession uses CHA. Nonetheless, to the best of our knowledge, its significance in the field of endodontics has not been assessed so far.
In the field of bone regeneration and replacement, calcium phosphates are among the materials most frequently used in dentistry and medical applications. HA is the most commonly utilized calcium-phosphate substance. Types A and B are the two primary forms of CHA, resulting from hydroxyl substitution and phosphate substitution, respectively (21,22).Type B CHA was employed in this study. According to Landi et al, the carbonates in the Type B HA network caused a reduction in crystallinity, which increased the biomaterial’s solubility both in vitro and in vivo and the amount of neobone formation (23). The non-sintered, non-crystalline CHA’s antibacterial potential was assessed in the current investigation.
Antimicrobial testing was conducted using three distinct concentrations (0.05 M, 0.1 M, and 0.5 M). The concentration ranged from 0.05 M at the lowest to 0.5 M at the maximum. Selecting three distinct concentrations of material enables a thorough investigation of its effects and assists in process optimization. In our study, the antibacterial activity of CHA was compared with that of MTA, a widely used pulp capping agent. Due to its exceptional biocompatibility, MTA can replace injured pulp without the need for a root canal by promoting the growth of hard tissue that serves as a barrier and maintains the pulp’s vitality (24). According to research by Viswanath et al, MTA Plus had statistically stronger antifungal efficacy against C. albicans (P < 0.05) than Biodentine and EndoSequence root repair material (25). The findings of the study by Atom et al demonstrated that freshly mixed MTA-Angelus has superior antimicrobial activity against E. faecalis when compared to Dycal (3). Another investigation by Ravindran et alrevealed that the newly modified MTA exhibited superior antibacterial and antifungal activity against E. faecalis, S. mutans, and C. albicans compared to both conventional MTA and Biodentine (26). The antibacterial properties of MTA are primarily due to its key constituents, tricalcium silicate, and dicalcium silicate. Upon hydration, these components form an alkaline calcium silicate gel. This gel releases calcium hydroxide, which subsequently produces hydroxyl ions, leading to a highly alkaline environment. This elevated pH inhibits microbial proliferation (12).
In contrast, the antibacterial activity of TheraCal LC, MTA, Biodentine, and Dycal following setting reactions against S. mutans, Lactobacillus acidophilus, and E. faecalis was shown in research by Akin et al. When the setting process was finished, the studied pulp-capping materials showed no antibacterial activity. There was a small zone of inhibition against S. mutans only in Dycal (27).In contradistinction to the results obtained in this study, Estrela et al demonstrated in their investigation that MTA lacked antibacterial efficacy against E. faecalis (28). The results of Esteki et al revealed that Biodentine exhibited a more potent antimicrobial effect against E. faecalis and C. albicans compared to MTA and calcium-enriched mixture cement (29).
According to a study by Resmim et al, evaluating the antibacterial effect of HAs, bacteria grown with HA formed fewer colonies than the control (bacteria cultured without the tested material), suggesting that HA may have an inhibitory effect on S. aureus growth. While materials prepared from bovine bones at these temperatures represented a growth inhibitory impact of 30% and 81%, respectively, the HA formed by calcining pig bones at 850°C and 1000°C could block 38% and 56% of the bacterial cells (30). The results of another study by Muhammad et al indicated that Meso-CHA has little antibacterial activity, in contrast to the previous study on the antimicrobial ability of HA (15).
The enhanced antimicrobial property of 0.1 M CHA against C. albicans and E. faecalis could be attributed to its unique chemical composition and surface characteristics.
Nanoparticles can induce antibacterial effects through various mechanisms, such as modifying the cell wall and cytoplasm, altering membrane integrity, or inhibiting respiratory activity (31). The antimicrobial activity of HA is due to the generation of reactive oxygen species (OH−, H2O2, and O2−2) on the surface of the HA nanoparticles, which cause lethal damage to the bacteria (30). The carbonation of HA alters its physicochemical properties, potentially increasing its surface reactivity and facilitating stronger interactions with microbial cells. At higher concentrations (0.5 M), the CHA might reach a saturation point, where its antimicrobial properties plateau or even diminish. This saturation effect could occur due to the saturation of active sites on the material’s surface, limiting its ability to interact with and inhibit microbial growth effectively.
The present research explored the antimicrobial potential of CHA, a biomaterial traditionally used in bone regeneration. While the antimicrobial properties of HA have been extensively studied, the focus on its carbonated form has been comparatively limited. This study specifically targeted three clinically relevant oral pathogens, namely, S. mutans, E. faecalis, and C. albicans. These microorganisms play a pivotal role in the pathogenesis of dental caries and endodontic infections. By focusing on these specific bacteria and fungi, the research directly addressed critical clinical challenges associated with oral health and provided valuable insights into the antimicrobial properties of CHA and its potential use as a pulp capping agent. The findings of this study can contribute to the development of novel and effective HA-based materials for VPT. This study is the first to evaluate the antimicrobial properties of CHA at different concentrations and compare them with those of other pulp capping agents. However, it is essential to acknowledge the limitations of this study, including its in vitro nature, which may not directly translate to clinical settings. Further research is warranted to explore the clinical efficacy and safety of CHA in endodontic procedures.
Conclusion
The antimicrobial property of a pulp capping material helps reduce the risk of secondary infections and supports the overall success of pulp capping procedures. When used at 0.1 M, carbonated HA had strong antibacterial activity against C. albicans. Antimicrobial activity against S. mutansand E. faecalis was demonstrated at 0.5 M and 0.1 M. CHA exhibited significant antimicrobial activity against various pathogens at different concentrations, suggesting its potential utility as a pulp-capping agent.
Authors’ Contribution
Conceptualization: S. Swathi Priyadharshini.
Data curation: Anand Sherwood, Chinnsamy Ragavendran.
Formal analysis: Anand Sherwood, Chinnsamy Ragavendran.
Investigation: S. Swathi Priyadharshini.
Methodology: Chinnasamy Ragavendran.
Project administration: Chinnasamy Ragavendran, S. Swathi Priyadharshini.
Resources: Rathna Piriyanga.
Supervision: Chinnasamy Ragavendran.
Validation: Anand Sherwood, S. Swathi Priyadharshini.
Visualization: Rathna Piriyanga.
Writing–original draft: S. Swathi Priyadharshini.
Writing–review & editing: S. Swathi Priyadharshini, Rathna Piriyanga.
Competing Interests
There is no conflict of interests.
Ethical Approval
The study protocol was reviewed and authorized by an Institutional Review Board (Saveetha Dental College and Hospitals (SIMATS)) with reference number SRB/SDC/PhD/ENDO-2309/23/TH-081.
Funding
Nil.
References
- Blancas B, de Lourdes Lanzagorta M, Jiménez-Garcia LF, Lara R, Molinari JL, Fernández AM. Study of the ultrastructure of Enterococcus faecalis and Streptococcus mutans incubated with salivary antimicrobial peptides. Clin Exp Dent Res 2021; 7(3):365-75. doi: 10.1002/cre2.430 [Crossref] [ Google Scholar]
- Islam R, Islam MR, Tanaka T, Alam MK, Ahmed HM, Sano H. Direct pulp capping procedures - evidence and practice. Jpn Dent Sci Rev 2023; 59:48-61. doi: 10.1016/j.jdsr.2023.02.002 [Crossref] [ Google Scholar]
- Atom J, Devi NR, Lairenlakpam R, Dafer Al Wadei MH, Hakami AR, BinShaya AS. Antimicrobial efficacy of different pulp-capping materials against Enterococcus faecalis: an in vitro study. J Pharm Bioallied Sci 2021; 13(Suppl 1):S608-11. doi: 10.4103/jpbs.JPBS_586_20 [Crossref] [ Google Scholar]
- Franzin NR, Sostena M, dos Santos AD, Moura MR, de Camargo ER, Hosida TY. Novel pulp capping material based on sodium trimetaphosphate: synthesis, characterization, and antimicrobial properties. J Appl Oral Sci 2022; 30:e20210483. doi: 10.1590/1678-7757-2021-0483 [Crossref] [ Google Scholar]
- Bergara-Muguruza L, Mäkelä K, Yrjälä T, Salonen J, Yamashita K, Nakamura M. Surface electric fields increase human osteoclast resorption through improved wettability on carbonate-incorporated apatite. ACS Appl Mater Interfaces 2021; 13(49):58270-8. doi: 10.1021/acsami.1c14358 [Crossref] [ Google Scholar]
- Zhang Y, Yin QS, Zhao HF, Li J, Wei YT, Cui FZ. Antibacterial and biological properties of silver-loaded coralline hydroxyapatite. Front Mater Sci China 2010; 4(4):359-65. doi: 10.1007/s11706-010-0112-2 [Crossref] [ Google Scholar]
- Sukmawati AN, Pramestri S, Soesilowati AS, Suryono S. Carbonated hydroxyapatite containing propolis as an antibacterial agent candidate against Aggregatibacteractinomycetemcomitans. Trad Med J 2020; 25(3):196-200. doi: 10.22146/mot.62712 [Crossref] [ Google Scholar]
- Priyadharshini SS, Ahmed SA, Savadamoorthi KS. In vitro antibacterial effectiveness of three different dentin bonding systems against Streptococcus mutans and Enterococcus faecalis. J Int Oral Health 2017; 9(1):33-7. doi: 10.4103/jioh.jioh_2_16 [Crossref] [ Google Scholar]
- Delboni MG, Gomes BP, Francisco PA, Teixeira FB, Drake D. Diversity of Enterococcus faecalis genotypes from multiple oral sites associated with endodontic failure using repetitive sequence-based polymerase chain reaction and arbitrarily primed polymerase chain reaction. J Endod 2017; 43(3):377-82. doi: 10.1016/j.joen.2016.10.042 [Crossref] [ Google Scholar]
- Gaeta C, Marruganti C, Ali IA, Fabbro A, Pinzauti D, Santoro F. The presence of Enterococcus faecalis in saliva as a risk factor for endodontic infection. Front Cell Infect Microbiol 2023; 13:1061645. doi: 10.3389/fcimb.2023.1061645 [Crossref] [ Google Scholar]
- Ghasempour M, Sefidgar SA, Eyzadian H, Gharakhani S. Prevalence of candida albicans in dental plaque and caries lesion of early childhood caries (ECC) according to sampling site. Caspian J Intern Med 2011; 2(4):304-8. [ Google Scholar]
- Lim M, Yoo S. The antibacterial activity of mineral trioxide aggregate containing calcium fluoride. J Dent Sci 2022; 17(2):836-41. doi: 10.1016/j.jds.2021.09.005 [Crossref] [ Google Scholar]
- Asgary S, Akbari Kamrani F, Taheri S. Evaluation of antimicrobial effect of MTA, calcium hydroxide, and CEM cement. Iran Endod J 2007; 2(3):105-9. [ Google Scholar]
- Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod 1999; 25(3):197-205. doi: 10.1016/s0099-2399(99)80142-3 [Crossref] [ Google Scholar]
- Mohammad NF, Fadzli FS, Saleh SS, Mohamad CW, Taib MA. Antibacterial ability of mesoporous carbonated hydroxyapatite. J Phys Conf Ser 2019; 1372(1):012081. doi: 10.1088/1742-6596/1372/1/012081 [Crossref] [ Google Scholar]
- Devitaningtyas N, Syaify A, Herawati D, Suryono S. Evaluation of antibacterial potential of carbonated hydroxyapatite combined with propolis on Porphyromonasgingivalis. Trad Med J 2020; 25(1):55-8. doi: 10.22146/mot.55173 [Crossref] [ Google Scholar]
- Othman R, Mustafa Z, Loon CW, Mohd Noor AF. Effect of calcium precursors and pH on the precipitation of carbonated hydroxyapatite. Procedia Chem 2016; 19:539-45. doi: 10.1016/j.proche.2016.03.050 [Crossref] [ Google Scholar]
- Morita M, Kitagawa H, Nakayama K, Kitagawa R, Yamaguchi S, Imazato S. Antibacterial activities and mineral induction abilities of proprietary MTA cements. Dent Mater J 2021; 40(2):297-303. doi: 10.4012/dmj.2019-351 [Crossref] [ Google Scholar]
- Taha MY, Al-Shakir NM, Al-Sabawi NA. Antibacterial effect of dentin bonding agents: an in vitro study. Rafidain Dent J 2013; 13(2):228-34. doi: 10.33899/rden.2013.84778 [Crossref] [ Google Scholar]
- Bhardwaj A, Bhardwaj A, Rao N. Evaluation of antifungal activity of white-colored mineral trioxide aggregate on different strains of Candida albicans in vitro. J Conserv Dent 2014; 17(3):276-9. doi: 10.4103/0972-0707.131799 [Crossref] [ Google Scholar]
- Resende RF, Sartoretto SC, Uzeda MJ, Alves A, Calasans-Maia JA, Rossi AM. Randomized controlled clinical trial of nanostructured carbonated hydroxyapatite for alveolar bone repair. Materials (Basel) 2019; 12(22):3645. doi: 10.3390/ma12223645 [Crossref] [ Google Scholar]
- Habibovic P, Juhl MV, Clyens S, Martinetti R, Dolcini L, Theilgaard N. Comparison of two carbonated apatite ceramics in vivo. Acta Biomater 2010; 6(6):2219-26. doi: 10.1016/j.actbio.2009.11.028 [Crossref] [ Google Scholar]
- Landi E, Celotti G, Logroscino G, Tampieri A. Carbonated hydroxyapatite as bone substitute. J Eur Ceram Soc 2003; 23(15):2931-7. doi: 10.1016/s0955-2219(03)00304-2 [Crossref] [ Google Scholar]
- Jain AS, Gupta AS, Agarwal R. Comparative evaluation of the antibacterial activity of two biocompatible materials ie Biodentine and MTA when used as a direct pulp capping agent against Streptococcus mutans and Enterococcus faecalis- an in vitro study. Endodontology 2018; 30(1):66-8. doi: 10.4103/endo.endo_66_17 [Crossref] [ Google Scholar]
- Viswanath G, Tilakchand M, Naik BD, Kalabhavi AS, Kulkarni RD. Comparative evaluation of antimicrobial and antifungal efficacy of bioactive root-end filling materials: an in vitro study. J Conserv Dent 2021; 24(2):148-52. doi: 10.4103/jcd.jcd_548_19 [Crossref] [ Google Scholar]
- Ravindran V, Jeevanandan G. Comparative evaluation of the physical and antimicrobial properties of mineral trioxide aggregate, Biodentine, and a modified fast-setting mineral trioxide aggregate without tricalcium aluminate: an in vitro study. Cureus 2023; 15(8):e42856. doi: 10.7759/cureus.42856 [Crossref] [ Google Scholar]
- Akin D, Ateş M, Atalayin Özkaya Ç. Antibacterial activity of different pulp capping materials after completed setting reaction. Ege Üniv Diş Hekim Fak Derg 2023; 44(2):109-15. doi: 10.5505/eudfd.2023.24392 [Crossref] [ Google Scholar]
- Estrela C, Bammann LL, Estrela CR, Silva RS, Pécora JD. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J 2000; 11(1):3-9. [ Google Scholar]
- Esteki P, Zare Jahromi M, Tahmourespour A. In vitro antimicrobial activity of mineral trioxide aggregate, Biodentine, and calcium-enriched mixture cement against Enterococcus faecalis, Streptococcus mutans, and Candida albicans using the agar diffusion technique. Dent Res J (Isfahan) 2021; 18:3. [ Google Scholar]
- Resmim CM, Dalpasquale M, Vielmo NIC, Mariani FQ, Villalba JC, Anaissi FJ. Study of physico-chemical properties and in vitro antimicrobial activity of hydroxyapatites obtained from bone calcination. Prog Biomater 2019; 8(1):1-9. doi: 10.1007/s40204-018-0105-2 [Crossref] [ Google Scholar]
- Lamkhao S, Phaya M, Jansakun C, Chandet N, Thongkorn K, Rujijanagul G. Synthesis of hydroxyapatite with antibacterial properties using a microwave-assisted combustion method. Sci Rep 2019; 9(1):4015. doi: 10.1038/s41598-019-40488-8 [Crossref] [ Google Scholar]