Avicenna J Dent Res. 16(2):70-76.
doi: 10.34172/ajdr.1793
Original Article
Locally-Delivered 1% Metformin Gel as an Adjunct to Scaling and Root Planning in the Treatment of Severe Chronic Periodontitis: A Randomized, Controlled Clinical Trial
Parvin Arbabi Kalati 1  , Mohammad Hossein Foroutani 2
, Mohammad Hossein Foroutani 2  , Elaheh Karami 3, *
, Elaheh Karami 3, * 
Author information:
1Department of Periodontics Dental School, Zahedan University of Medical Sciences, Zahedan, Iran
2General Dentist, Dental Clinic, Robat Karim, Iran
3Periodontist, Periodontics, Canada
Abstract
Background: Periodontitis is a chronic inflammatory disease that affects the teeth’s supporting structures, leading to clinical attachment loss, pocket formation, and tooth loss if left untreated. Scaling and root planning (SRP) is the gold standard for removing bacterial biofilm and calculus from tooth surfaces. However, in most situations, including tooth fractures, there is a need for adjunctive therapies to complement and improve treatment outcomes. This study aimed to evaluate the efficacy of locally delivered metformin (MF) 1% gel as an adjunct to SRP in treating severe chronic periodontitis.
Methods: A total of 36 volunteers were randomly assigned to two treatment groups, namely, SRP plus placebo gel and SRP plus 1% MF gel. Clinical parameters such as pocket probing depth (PD), clinical attachment level (CAL), and gingival recession (GR) were recorded at baseline, 2, and 4 months. The data were analyzed using independent T-tests, one-way, and repeated measures analysis of variance (ANOVA) using SPSS 24 software.
Results: All groups exhibited improvements in periodontal parameters such as PD and CAL. While the mean reductions in PD and CAL were not statistically significant after 3 weeks of treatment between the two groups (P=0.193), the MF group demonstrated significantly greater improvements in PD, measuring 3.49 mm compared to 1.87 mm in the control group (P=0.007), as well as in CAL, which measured 2.98 mm versus 1.72 mm in the control group (P=0.014).
Conclusion: The adjunctive use of locally delivered 1% MF gel could stimulate a significant reduction in PD and increases in CAL compared to the placebo gel, while there was no GR resulting from the local delivery of the drug. This suggests that the MF gel may offer benefits in the treatment of severe chronic periodontitis by enhancing periodontal healing without inducing GR.
Keywords: Drug delivery systems, Severe chronic periodontitis, Metformin, Periodontal pocket
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Arbabi kalati P, Foroutani MH, Karami E. Locally-delivered 1% metformin gel as an adjunct to scaling and root planning in the treatment of severe chronic periodontitis: a randomized, controlled clinical trial. Avicenna J Dent Res. 2024; 16(2):70-76. doi:10.34172/ajdr.1793
Background
One of the most common long-term inflammatory conditions is periodontitis, recognized by inflammation and destruction of tooth-supporting tissue, leading to tooth loss if left untreated (1). Although scaling and root planning (SRP) has been known as the gold standard for the treatment of periodontal diseases (2), specific physical limitations, such as furcations, deep periodontal pockets, or interproximal regions of misaligned teeth, may result in an incomplete diminution of anaerobic infection and augment the likelihood of recurrence (3). Hence, local and systemic prescriptions of antibiotics, photodynamic therapies such as laser therapy, and bisphosphonates have been suggested to complement SRP treatment. These adjunctive approaches lower the number of bacteria and improve clinical periodontal parameters, such as reducing probing depth (PD) and enhancing clinical attachment level (CAL) (4-7). The utilization of local drug delivery systems has been most popular over the last three decades to complement SRP in periodontal therapy. This system allows drugs to significantly exceed the minimum inhibitory concentration and persist for up to several weeks, resulting in higher efficacy and fewer side effects by regulating drug discharge (8).
In recent years, metformin (MF), which is the first line of treatment for type 2 diabetes, has been investigated for the treatment of periodontal bone defects in chronic periodontitis (9). MF has shown various therapeutic effects in animals and laboratory studies related to periodontitis. These effects include reducing oxidative stress, inflammatory reactions, and bone loss in the rat models of periodontitis (10,11), as well as decreasing cytokine production in human cells stimulated with lipopolysaccharide (12,13). MF reduces pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha in gingival fibroblast cells by affecting activating transcription factor-3 and nuclear factor kappaB expression (14). It also lowers IL-18 and IL-1β levels in periodontal ligament cells by inhibiting the nucleotide-binding domain, leucine-rich-containing family, and pyrin domain-containing-3 inflammasome and reducing caspase-1 expression (15). Furthermore, MF enhances autophagy in periodontal ligament cells by increasing adenosine monophosphate-activated protein kinase activation and decreasing P16 and P21 expression, exhibiting anti-senescence effects (13). In terms of osteogenic effects, MF upregulates the expression of osteogenic genes such as alkaline phosphatase and osteocalcin via adenosine monophosphate-activated protein kinase activation (16). Additionally, it promotes osteoblastic differentiation and inhibits osteoclastic differentiation by increasing osteoprotegerin expression and decreasing receptor activator of nuclear factor κ expression (10).
While preclinical research has established effects that offer promising implications for clinical practice and further research in periodontal therapy, there is optimism regarding the therapeutic effectiveness of MF in reducing PD and increasing clinical attachment in patients with severe chronic periodontitis. However, clinical studies employing MF gel delivery into periodontal pockets are limited, prompting the need for further investigation. Considering the above-mentioned explanations, the present study was designed as a randomized clinical trial to evaluate the effectiveness of the 1% MF gel as a local drug delivery along with SRP.
Materials and Methods
Sample Selection
The present study was a randomized controlled clinical trial that included 36 patients (18 men and 18 women with a mean age of 41 years) diagnosed with severe chronic periodontitis from the Department of Periodontology, Faculty of Dentistry, Zahedan University of Medical Sciences, Iran. The following formula was used to determine the sample size.
where δ and ε represent the clinically significant margin and the actual difference. n1 = kn2, respectively, and
considering the following values (17), α = 0.05, β = 0.05, k = 1.
First, ethical approval was obtained from the National Research Ethics Committee of Iran, and then oral and written consent was obtained from all participants. Sample selection criteria included individuals with peri-implantitis definition ≥ 6 mm and CAL ≥ 5 mm, known as severe chronic forms of periodontitis (18), no prior history of antibiotic usage or periodontal therapy within the past 6 months, and no history of smoking or tobacco use.
On the other hand, patients were excluded if they had definite systemic diseases, a definite or possible allergy to the MF group, undergone systemic antidiabetic or MF treatment, suffered from immunodeficiency, and were pregnant or lactated.
A stratified random allocation method was utilized to assign patients to the study groups. Initially, the patients were stratified based on age and gender, and subsequently, they were placed into one of the study groups through pairwise permutations.
Metformin Gel Preparation
To prepare the MF gel, we followed the method outlined by Mohapatra et al (19). Dry gel gum powder was dispersed and stirred in distilled water heated to 95 °C for 20 minutes using a magnetic stirrer. Then, a certain quantity of mannitol was added to the gellan gum solution with constant stirring, maintaining the temperature above 80 °C. Next, a measured amount of MF, sucralose, citric acid, and preservatives (methylparaben and propylparaben) were added, respectively, with stirring. Finally, the required amount of sodium citrate dispersed in 10 mL of distilled water was added to the mixture. The gel formed as the mixture cooled to room temperature and was prepared at a concentration of 1%.
Study Protocol
All patients underwent full-mouth SRP conducted by a single examiner. To standardize oral hygiene, the patients were provided with an Oral-B medium toothbrush and Signal Complete toothpaste, along with instructions on proper brushing techniques using the Bass method. Following non-surgical debridement, three dental sites meeting the inclusion criteria were treated with 10 mL of the 1% MF gel in the experimental group and a placebo gel in the control group. Gel application into the periodontal pockets was performed using a blunt cannula inserted into the base of the pockets. To ensure blinding in the study, a placebo gel was formulated to replicate the composition of the MF gel, excluding the active ingredient, to match its appearance, texture, and taste. Both gels were stored in identical syringes, thus keeping both the examiner and the patient unaware of the type of gel used and maintaining blindness. After treatment, no anti-inflammatory drugs or antibiotics were prescribed for the patients. Patients were instructed to refrain from brushing, flossing in the treated areas, and chewing hard or sticky foods for one week. To investigate the side effects of MF, not only were oral examinations performed to detect any sores, discoloration, and irritation, but patients were also queried about the occurrence of medical complications related to MF side effects, including nausea, vomiting, diarrhea, altered taste in the mouth, decreased appetite, and persistent abdominal pain, at each follow-up session. The observed supragingival deposits were also removed.
Clinical parameters, including PD, CAL, and gingival recession (GR), were recorded at baseline (before SRP) and after 2 months. At the two-month recall session, the target areas were retreated with the MF gel and a placebo for the second time. The clinical parameters were then measured again in the fourth month.
Statistical Analysis
All data related to clinical parameters were tabulated and statistically analyzed using SPSS24 software. The assumption of normality was checked using the Kolmogorov-Smirnov test. After confirming the normal distribution of data, intergroup analysis was performed using unpaired or independent t-tests. Intragroup analysis was also conducted using a one-way analysis of variance (ANOVA) with Bonferroni’s post hoc test. Additionally, a repeated-measures ANOVA was performed to explore the interaction effects between treatment groups and time intervals on the measured parameters. Repeated-measures ANOVA also allowed for the assessment of changes for all parameters at all time intervals with a within-subject effect.
Results
Thirty-six patients who completed the study reported no adverse complications during the follow-up, and their soft tissues healed normally (Figure 1). Initially, no statistically meaningful difference was observed in the mean age and gender distribution (P = 0.260 and P = 0.317, respectively, Table 1). Both groups showed remarkable improvements in clinical parameters, including PD and CAL, at all visits, exhibiting comparable levels of oral and dental hygiene between the two groups during the study. However, according to the t-test, while the clinical parameters of PD and CAL did not reveal significant differences between both groups at the beginning and 3 weeks (P = 0.193), the MF group exhibited significantly greater improvements in PD (3.49 mm vs. 1.87 mm, P = 0.007) and CAL (2.98 mm vs. 1.72 mm, P = 0.014), the results of which are presented in Tables 2 and 3.
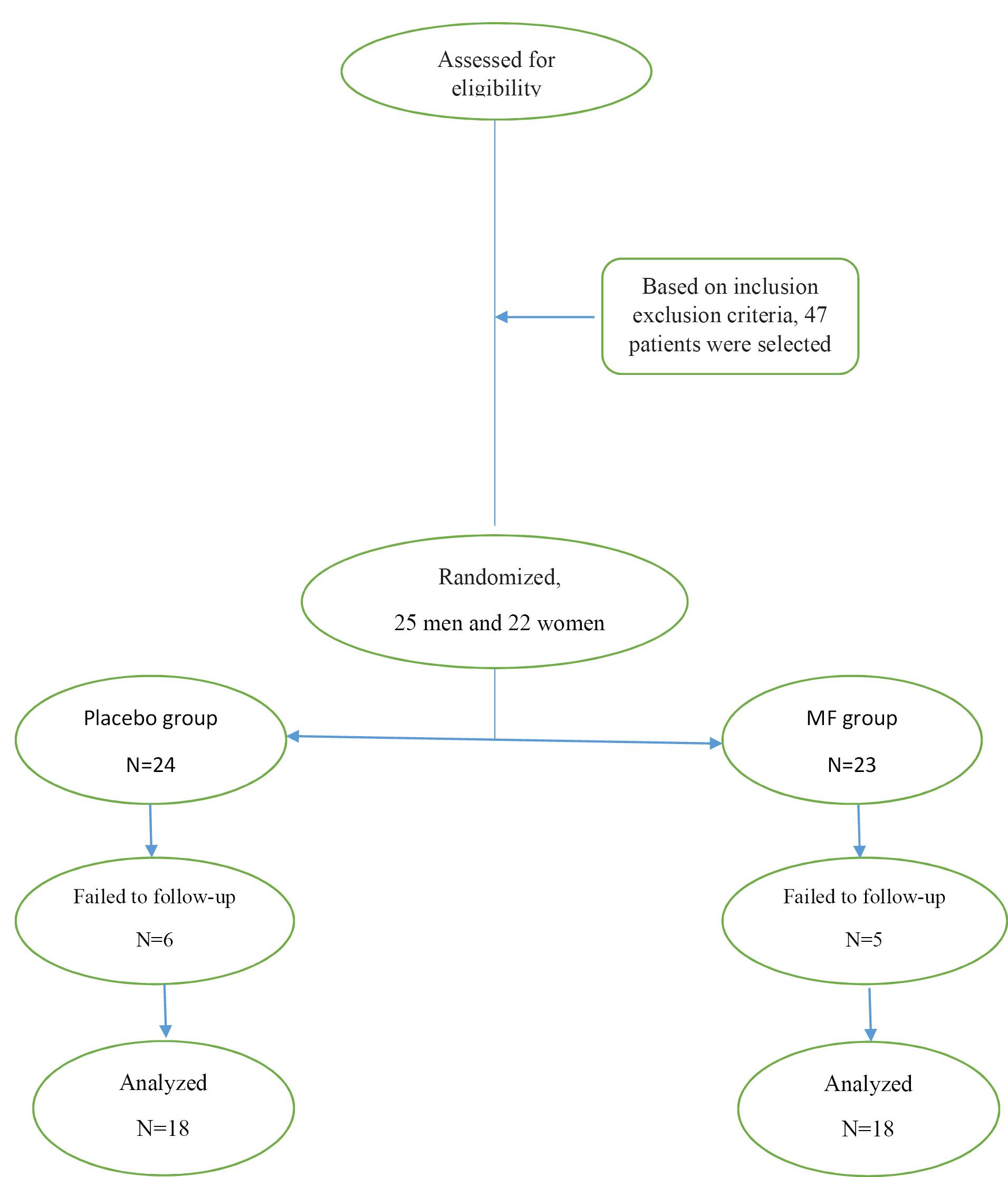
Figure 1.
Consort Flowchart
.
Consort Flowchart
Table 1.
Basic Demographic Characteristics of the Study
|
Parameter
|
Placebo Group
|
MF Group
|
P
Value
|
| Number of patients |
18 |
18 |
|
| Mean age (years) ± SD |
42.94 ± 11.94 |
38.34 ± 12.23 |
0.260a |
| Age (years) |
22-63 |
20-61 |
|
| Male/female |
10/8 |
9/9 |
0.317b |
Note. SD: Standard deviation.
aIndependent t-test; bChi-square test.
Table 2.
Clinical Parameters for MF and Placebo Groups (Mean ± SD) at Different Time Intervals (in mm)
|
Parameter
|
Visit
|
Placebo Group (mean±SD)
|
MF Group (mean±SD)
|
P
Valueb
|
| PD |
Baseline |
6.79 ± 1.53 |
7.33 ± 1.96 |
0.366 |
| 3 Weeks |
5.70 ± 1.38 |
5.16 ± 1.025 |
0.193 |
| 2 Months |
5.29 ± 1.32 |
4.35 ± 0.97 |
0.019 |
| 4 Months |
4.92 ± 1.32 |
3.83 ± 0.96 |
0.007 |
|
P valuea |
|
> 0.001 |
> 0.001 |
|
| CAL |
Baseline |
6.35 ± 0.77 |
6.68 ± 0.81 |
0.214 |
| 3 Weeks |
5.11 ± 1.17 |
4.85 ± 1.25 |
0.524 |
| 2 Months |
4.96 ± 1.05 |
4.16 ± 1.18 |
0.041 |
| 4 Months |
4.63 ± 1.01 |
3.71 ± 1.14 |
0.014 |
|
P valuea |
|
> 0.001 |
> 0.001 |
|
| GR |
Baseline |
0.52 ± 0.69 |
0.65 ± 0.78 |
0.602 |
| 3 Weeks |
0.57 ± 0.72 |
0.66 ± 0.77 |
0.712 |
| 2 Months |
0.63 ± 0.77 |
0.66 ± 0.77 |
0.780 |
| 4 Months |
0.65 ± 0.77 |
0.72 ± 0.79 |
0.777 |
|
P valuea |
|
0.206 |
0.319 |
|
Note. SD: Standard deviation; PD: Probing depth; CAL: Clinical attachment level; GR: Gingival recession; MF: Metformin; ANOVA: Analysis of variance.
aOne-way repeated measures ANOVA, bIndependent t-test.
Table 3.
Comparison of F the Beginning to the 4th Month After Scaling and Root Planning
|
Clinical Parameters |
Group |
Mean |
Standard Deviation |
Test Statistic |
P Valuea |
| PD |
Experimental |
-3.49 |
1.24 |
|
|
| Control |
-1.87 |
0.64 |
4.946 |
0.001 > |
| GR |
Experimental |
+ 0.07 |
0.18 |
|
|
| Control |
+ 0.13 |
0.23 |
0.798 |
0.431 |
| CAL |
Experimental |
-2.98 |
0.96 |
|
|
| Control |
-1.72 |
0.55 |
4.838 |
0.001 > |
Note. PD: Probing depth; CAL: Clinical attachment level; GR: Gingival recession.
**Independent t-test.
A one-way ANOVA with repeated measurements represented a significant reduction in mean CAL and PD in both groups across different stages of the study. Additionally, a two-by-two comparison of PD values using the Bonferroni test demonstrated a significant decrease in both groups, with a significant reduction in CAL in the MF-treated group. However, no significant difference was found in the control group from the third week to the second month after treatment. Furthermore, the mean gingival recession gradually increased during the study; however, there were no statistically significant differences in intragroup and intergroup comparisons (P > 0.05). The repeated measures ANOVA for PD, CAL, and GR showed a significant interaction effect between time and group (P < 0.0001), indicating that the changes in all clinical parameters over time were significantly different between the control and MF groups.
Generally, according to the independent T-test, the average PD and CAL in both groups decreased from the beginning to the end of the study; however, the reduction in PD and CAL in the MF group was significantly greater (P < 0.001). Moreover, the increased GR in both groups demonstrated no significant difference (P < 0.05).
Figures 2, 3, and 4 illustrate mean changes in PD, CAL, and GR for both groups, respectively.
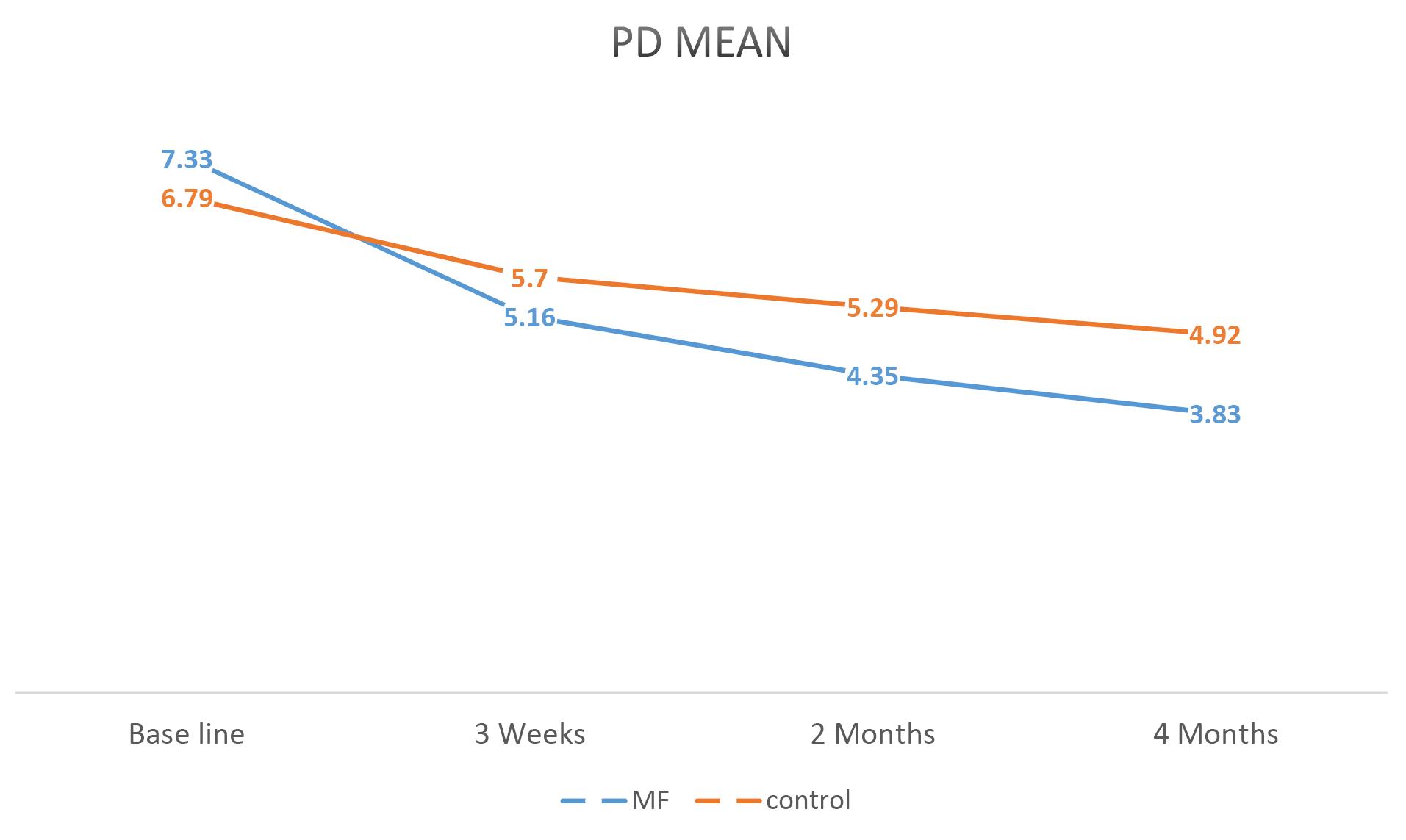
Figure 2.
A Linear Graph Comparing the Average PD in the MF and Control Groups During the Study. Note. PD: Probing depth; MF: Metformin
.
A Linear Graph Comparing the Average PD in the MF and Control Groups During the Study. Note. PD: Probing depth; MF: Metformin
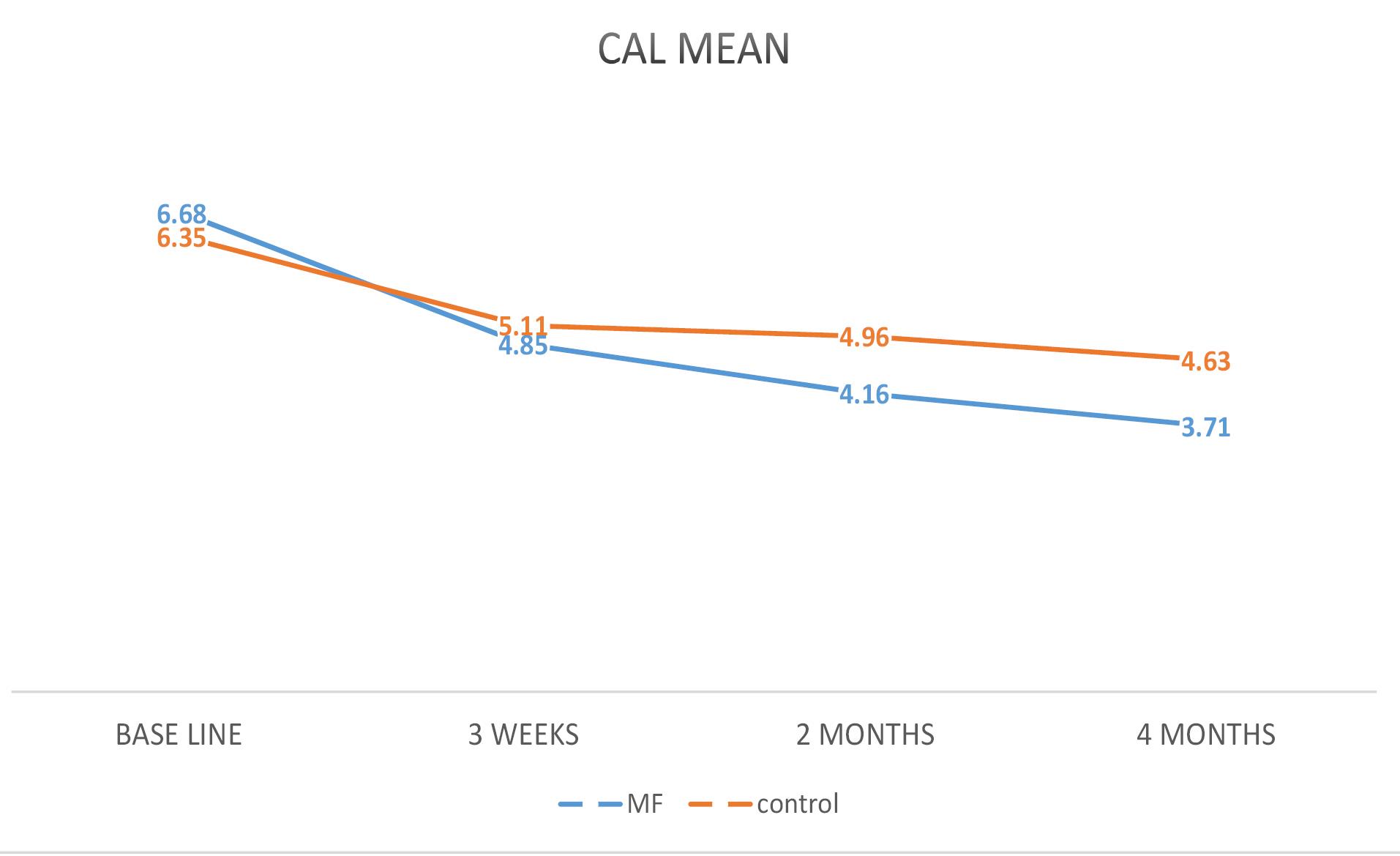
Figure 3.
A Linear Graph Comparing the Average CAL in the MF and Control Groups During the Study. Note. CAL: Clinical attachment level; MF: Metformin
.
A Linear Graph Comparing the Average CAL in the MF and Control Groups During the Study. Note. CAL: Clinical attachment level; MF: Metformin
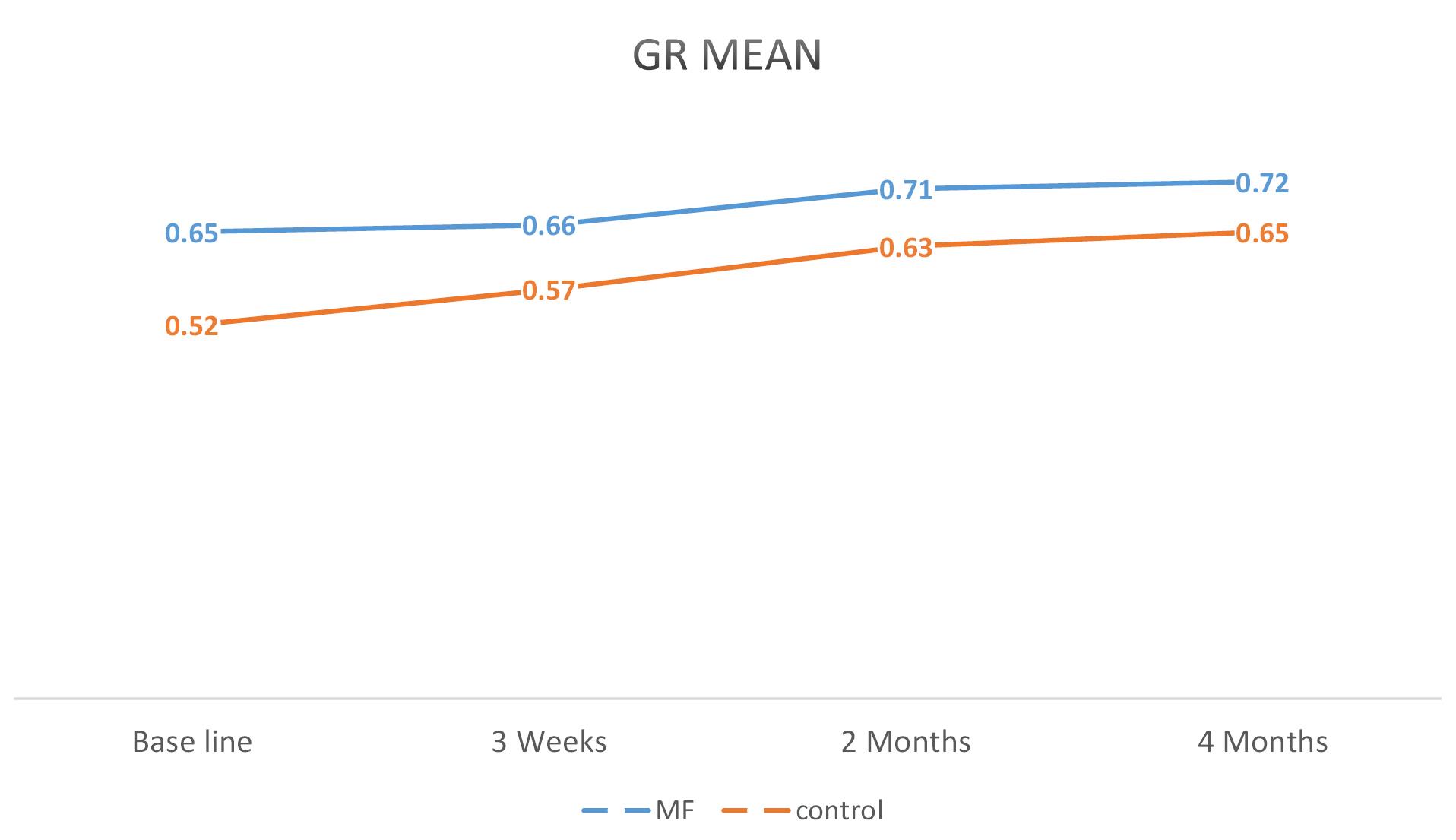
Figure 4.
A Linear Graph Comparing the Average GR in the MF and Control Groups During the Study. Note. GR: Gingival recession; MF: Metformin
.
A Linear Graph Comparing the Average GR in the MF and Control Groups During the Study. Note. GR: Gingival recession; MF: Metformin
Discussion
The main factor driving the progression of periodontal disease is the host’s immune response to pathogens, involving the expression of cytokines, prostanoids, inflammatory cells, and osteoclast activation (20). Thus, to limit tissue destruction, modulating the inflammatory response with pharmacological interventions alongside eliminating pathogenic microbiota via SRP is crucial (21). Considering the therapeutic properties of MF, the current study assessed the clinical efficacy of the MF 1% gel locally derived post-SRP in patients with severe chronic periodontitis (CAL > 5 mm). The results confirmed superior improvements in clinical parameters compared to a placebo gel over 4 months.
In this clinical study, clinical parameters, including PD and CAL, as two pathognomonic parameters of periodontitis (22), as well as GR, were measured and recorded at the beginning. Then, the patients were treated with SRP and divided into the control (treatment with SRP and a placebo gel at the beginning and after two months) and experimental (treatment with SRP and subgingival use of the MF 1% gel at the beginning and after two months) groups. Finally, periodontal parameters were remeasured at 3-week, 2-month, and 4-month recall sessions. The results showed improvements in all clinical parameters at all time intervals in both groups, but no statistically significant difference was observed between the two groups until the third week. These findings align with those of previous studies. Grace and Malaiappan (23) reported the positive effects of the MF 1% gel on periodontal tissues over one month without significant comparison with the placebo. Additionally, intergroup analysis in our study revealed significant reductions in the mean PD and CAL scores between treatment groups in the second and fourth months. Similarly, in the study by Bashir et al, no significant change was found in pocket depth or gingival attachment after one month with the MF 1% gel, but significant improvements emerged at three and six months, which is consistent with our findings. Patil et al (24) also reported improvements in clinical parameters with MF 1.5% after three and six months. However, variations in the average reduction of pocket depth, clinical attachment gain, and ossification rate were observed among the studies. These differences may stem from variations in MF administration, release rate, study duration, combination with other agents, and study design. Regarding the study design, some studies employed open flap surgery for debridement, whereas our study utilized a non-invasive SRP approach (25-27). Furthermore, it is worth noting that the reduction in PD depends on the initial depth of the pocket (17), and periodontal bone healing remarkably occurs in the first 3 months post-treatment (28), indicating the necessity of longer treatment durations for MF to fully impact the tissues.
Pradeep et al conducted three studies analyzing MF concentration, treatment duration, the method of MF induction, and differences in initial pocket depth between treatment sites. In their first study, they delivered three MF gel concentrations (0.5%, 1%, and 1.5%) subgingivally to treat chronic periodontitis. They reported that although all concentrations reduced PD and CAL, the healing impact of the MF 1% concentration was more significant on intraosseous defects after 6 months, suggesting maximal clinical benefits at the minimal dosage (29). Hence, we adopted the MF 1% concentration in our study. In their second study, they demonstrated greater improvements in periodontal parameters after 9 months compared to a previous 6-month study, particularly in clinical attachment gain among sites with pocket depths ≥ 7 mm versus those ≥ 5 mm (9). In their third study, MF induction was administered in three stages (at baseline, for three months, and for six months) with similar results (17), which contradicts our study finding, where MF was induced in two stages.
In a similar project, Sreedhar et al (30) divided the test group into two subgroups based on MF induction timing, namely, one-step induction on the first day after scaling versus two-step induction on the first day and thirty days after scaling. Both subgroups showed periodontal parameter improvements after three months, with no significant difference in PD reduction. This highlights variations in treatment methods, particularly in the number of MF administrations and the time intervals for retreatment. In most studies, MF treatment was administered only at the study’s baseline, whereas in the present study, MF was subgingivally used at baseline and after two months.
The increase in clinical attachment by MF can be attributed to its antioxidant and anti-inflammatory properties and diminution of inflammatory cytokines, including IL-1 beta and 18 (15), and matrix metalloproteinase (MMP)-1, -2, and -8 (12). MMPs, by degrading type I and III collagens, lead to loss of tissue adhesion and the initiation of periodontal pockets (31). Additionally, the improvement in PD with topical MF use can be attributed to its osteogenic properties, including the stimulation of osteoblastic differentiation and inhibition of osteoclastic differentiation (10,16,32). Our findings conform to those of other studies, showing reduced pocket depth with subgingival MF use (9,17,25,33,34).
Local application of the MF gel in periodontal pockets offers sustained concentration, potentially boosting its anti-inflammatory and bone-protective effects while avoiding systemic side effects (9). However, drawbacks such as multiple applications, cost, local irritation, and patient compliance issues should be taken into consideration (35). Additionally, factors such as patient demographics, which may be influenced by genetic or environmental differences, and comorbidities such as systemic diseases, smoking habits, and immune responses, may influence the applicability of these results across different populations (24,36). Our study highlights the positive impact of topical MF on the most important clinical parameters of periodontitis, PD and CAL, conducted under completely blinded conditions, but acknowledges limitations such as a small sample size and specific inclusion criteria. Accordingly, future research with larger cohorts and longer durations, considering interference factors, is necessary to enhance generalizability.
Conclusion
In summary, the findings of this study demonstrated that the local delivery of the 1% MF gel in chronic periodontitis patients with baseline PD ≥ 6 mm and CAL ≥ 5 mm resulted in a significant enhancement in clinical parameters, including PD (3.49 mm) and CAL (2.98 mm) when compared to the placebo gel (1.87 mm and 1.72 mm, respectively) used adjunctively to SRP. MF offers a promising avenue for managing severe chronic periodontitis without the need for an aggressive approach. However, extended multicenter randomized, controlled clinical trials are necessary to assess the applicability of these findings to broader and more diverse patient populations. In addition, the simultaneous examination of clinical parameters and more sensitive biomarkers, such as IL, can yield stronger results.
Acknowledgments
The authors would like to thank the Zahedan University of Medical Sciences for financial support of this project.
Authors’ Contribution
Conceptualization: Parvin Arbabi Kalati.
Data curation: Mohammad Hossein Foroutani.
Formal analysis: Elahe Karami.
Investigation: Mohammad Hossein Foroutani.
Methodology: Parvin Arbabi Kalati.
Project administration: Parvin Arbabi Kalati.
Software: Mohammad Hossein Foroutani.
Resources: Elahe Karami.
Supervision: Parvin Arbabi Kalati.
Validation: Elahe Karami.
Visualization: Mohammad Hossein Foroutani.
Writing – original draft: Elahe Karami.
Writing – review & editing: Elahe Karami.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this study.
Ethical Approval
The study protocol, with an IRCT registration number of IRCT20200623047900N1, was approved by the Ethics Committee of Zahedan University of Medical Sciences.
Funding
This study was funded by Zahedan University of Medical Sciences.
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016.
Lancet. 2017;390(10100):1211-59. 10.1016/s0140-6736(17)32154-2.
- Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000 2002; 28:12-55. doi: 10.1034/j.1600-0757.2002.280102.x [Crossref] [ Google Scholar]
- Cobb CM. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol 2002; 29 Suppl 2:6-16. [ Google Scholar]
- Zandbergen D, Slot DE, Cobb CM, Van der Weijden FA. The clinical effect of scaling and root planing and the concomitant administration of systemic amoxicillin and metronidazole: a systematic review. J Periodontol 2013; 84(3):332-51. doi: 10.1902/jop.2012.120040 [Crossref] [ Google Scholar]
- Akram Z, Abduljabbar T, Kellesarian SV, Abu Hassan MI, Javed F, Vohra F. Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: a systematic review. Br J Clin Pharmacol 2017; 83(3):444-54. doi: 10.1111/bcp.13147 [Crossref] [ Google Scholar]
- Vohra F, Akram Z, Safii SH, Vaithilingam RD, Ghanem A, Sergis K. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: a systematic review. Photodiagnosis Photodyn Ther 2016; 13:139-47. doi: 10.1016/j.pdpdt.2015.06.010 [Crossref] [ Google Scholar]
- Abduljabbar T, Javed F, Shah A, Samer MS, Vohra F, Akram Z. Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: a systematic review. Lasers Med Sci 2017; 32(2):449-59. doi: 10.1007/s10103-016-2086-5 [Crossref] [ Google Scholar]
- Wei Y, Deng Y, Ma S, Ran M, Jia Y, Meng J. Local drug delivery systems as therapeutic strategies against periodontitis: a systematic review. J Control Release 2021; 333:269-82. doi: 10.1016/j.jconrel.2021.03.041 [Crossref] [ Google Scholar]
- Pradeep AR, Patnaik K, Nagpal K, Karvekar S, Ramamurthy BL, Naik SB. Efficacy of locally-delivered 1% metformin gel in the treatment of intrabony defects in patients with chronic periodontitis: a randomized, controlled clinical trial. J Investig Clin Dent 2016; 7(3):239-45. doi: 10.1111/jicd.12150 [Crossref] [ Google Scholar]
- de Araújo AA, de Sousa Barbosa Freitas Pereira A, de Medeiros CA, de Castro Brito GA, de Carvalho Leitão RF, de Souza Araújo L. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS One 2017; 12(8):e0183506. doi: 10.1371/journal.pone.0183506 [Crossref] [ Google Scholar]
- Bak EJ, Park HG, Kim M, Kim SW, Kim S, Choi SH. The effect of metformin on alveolar bone in ligature-induced periodontitis in rats: a pilot study. J Periodontol 2010; 81(3):412-9. doi: 10.1902/jop.2009.090414 [Crossref] [ Google Scholar]
- Alshibani N, AlKattan R, Allam E, Alshehri FA, Shalabi MM, Almuhanna N. Effects of metformin on human gingival fibroblasts: an in vitro study. BMC Oral Health 2023; 23(1):292. doi: 10.1186/s12903-023-02978-0 [Crossref] [ Google Scholar]
- Kuang Y, Hu B, Feng G, Xiang M, Deng Y, Tan M. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology 2020; 21(1):13-27. doi: 10.1007/s10522-019-09838-x [Crossref] [ Google Scholar]
- Kang W, Wang T, Hu Z, Liu F, Sun Y, Ge S. Metformin inhibits Porphyromonasgingivalis lipopolysaccharide-influenced inflammatory response in human gingival fibroblasts via regulating activating transcription factor-3 expression. J Periodontol 2017; 88(10):e169-78. doi: 10.1902/jop.2017.170168 [Crossref] [ Google Scholar]
- Tan Y, Chen J, Jiang Y, Chen X, Li J, Chen B. The anti-periodontitis action of metformin via targeting NLRP3 inflammasome. Arch Oral Biol 2020; 114:104692. doi: 10.1016/j.archoralbio.2020.104692 [Crossref] [ Google Scholar]
- Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD, Kim DK. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone 2011; 48(4):885-93. doi: 10.1016/j.bone.2010.12.003 [Crossref] [ Google Scholar]
- Pradeep AR, Patnaik K, Nagpal K, Karvekar S, Guruprasad CN, Kumaraswamy KM. Efficacy of 1% metformin gel in patients with moderate and severe chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2017; 88(10):1023-9. doi: 10.1902/jop.2017.150096 [Crossref] [ Google Scholar]
- Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol 2018; 89 Suppl 1:S159-72. doi: 10.1002/jper.18-0006 [Crossref] [ Google Scholar]
- Mohapatra A, Parikh RK, Gohel MC. Formulation, development and evaluation of patient friendly dosage forms of metformin, part-II: oral soft gel. Asian J Pharm 2008; 2(3):172-6. doi: 10.22377/ajp.v2i3.207 [Crossref] [ Google Scholar]
- Champagne CM, Buchanan W, Reddy MS, Preisser JS, Beck JD, Offenbacher S. Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontol 2000 2003; 31:167-80. doi: 10.1034/j.1600-0757.2003.03110.x [Crossref] [ Google Scholar]
- Mushtaq I, Shukla P, Malhotra G, Dahiya V, Kataria P, Joshi CS. Comparative evaluation of 1% metformin gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis with scaling and root planing alone: a randomized controlled clinical trial. Int J Oral Care Res 2018; 6(2):79-88. [ Google Scholar]
- Preus HR, Gjermo P, Baelum V. A double-masked randomized clinical trial (RCT) comparing four periodontitis treatment strategies: 5-year clinical results. J Clin Periodontol 2017; 44(10):1029-38. doi: 10.1111/jcpe.12793 [Crossref] [ Google Scholar]
- Grace S, Malaiappan S. Effect of locally administered 1% metformin gel in the treatment of chronic periodontitis. J Pharm Sci Res 2017; 9(9):1463-5. [ Google Scholar]
- Patil KS, Mahajani M, Choudhary SH, Aldhuwayhi SD, Thakare A, Mustafa MZ. Efficacy of 15% metformin gel as an adjuvant to scaling, root planing, and curettage for the treatment of infrabony defects in chronic periodontitis patients. Contemp Clin Dent 2022; 13(1):18-23. doi: 10.4103/ccd.ccd_271_20 [Crossref] [ Google Scholar]
- Pradeep AR, Nagpal K, Karvekar S, Patnaik K, Naik SB, Guruprasad CN. Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2015; 86(6):729-37. doi: 10.1902/jop.2015.140646 [Crossref] [ Google Scholar]
- Khalifehzadeh S, Haghanifar S, Jenabian N, Kazemi S, Hajiahmadi M. Clinical and radiographic evaluation of applying 1% metformin biofilm with plasma rich in growth factor (PRGF) for treatment of two-wall intrabony periodontal defects: a randomized clinical trial. J Dent Res Dent Clin Dent Prospects 2019; 13(1):51-6. doi: 10.15171/joddd.2019.008 [Crossref] [ Google Scholar]
- Mitra DK, Donde RJ, Desai AB, Ghangrekar KP, Potdar PN, Shetty GP. A comparative study of demineralized freeze-dried bone allograft alone and with 1% metformin in the treatment of intrabony defects in patients with chronic periodontitis: a randomized clinical trial. J Indian Soc Periodontol 2023; 27(1):70-5. doi: 10.4103/jisp.jisp_628_21 [Crossref] [ Google Scholar]
- Sculean A, Stavropoulos A, Bosshardt DD. Self-regenerative capacity of intra-oral bone defects. J Clin Periodontol 2019; 46 Suppl 21:70-81. doi: 10.1111/jcpe.13075 [Crossref] [ Google Scholar]
- Pradeep AR, Rao NS, Naik SB, Kumari M. Efficacy of varying concentrations of subgingivally delivered metformin in the treatment of chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2013; 84(2):212-20. doi: 10.1902/jop.2012.120025 [Crossref] [ Google Scholar]
- Sreedhar A, Sikkander S, Pai J, Walvekar A, Baramappa R, Varkey A. Clinical and biochemical evaluation of efficacy of 1% metformin gel as adjunct to SRP in chronic periodontitis - a split mouth placebo-controlled study. Int J Sci Res 2022; 6(12):78-82. [ Google Scholar]
- Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res 2003; 82(2):82-90. doi: 10.1177/154405910308200202 [Crossref] [ Google Scholar]
- Zhang R, Liang Q, Kang W, Ge S. Metformin facilitates the proliferation, migration, and osteogenic differentiation of periodontal ligament stem cells in vitro. Cell Biol Int 2020; 44(1):70-9. doi: 10.1002/cbin.11202 [Crossref] [ Google Scholar]
- Rao NS, Pradeep AR, Kumari M, Naik SB. Locally delivered 1% metformin gel in the treatment of smokers with chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2013; 84(8):1165-71. doi: 10.1902/jop.2012.120298 [Crossref] [ Google Scholar]
- Kassem AA, Issa DA, Kotry GS, Farid RM. Thiolated alginate-based multiple layer mucoadhesive films of metformin forintra-pocket local delivery: in vitro characterization and clinical assessment. Drug Dev Ind Pharm 2017; 43(1):120-31. doi: 10.1080/03639045.2016.1224895 [Crossref] [ Google Scholar]
- Viglianisi G, Santonocito S, Lupi SM, Amato M, Spagnuolo G, Pesce P. Impact of local drug delivery and natural agents as new target strategies against periodontitis: new challenges for personalized therapeutic approach. Ther Adv Chronic Dis 2023; 14:20406223231191043. doi: 10.1177/20406223231191043 [Crossref] [ Google Scholar]
- Kurian IG, Dileep P, Ipshita S, Pradeep AR. Comparative evaluation of subgingivally-delivered 1% metformin and Aloe vera gel in the treatment of intrabony defects in chronic periodontitis patients: a randomized, controlled clinical trial. J Investig Clin Dent 2018; 9(3):e12324. doi: 10.1111/jicd.12324 [Crossref] [ Google Scholar]