Avicenna J Dent Res. 16(3):140-145.
doi: 10.34172/ajdr.1776
Original Article
BRCA1/2 Immunohistochemical Analysis in Unicystic Ameloblastoma, Multicystic Ameloblastoma, and Ameloblastic Carcinoma
Soussan Irani 1, 2, *  , Mitra Rafizadeh 3
, Mitra Rafizadeh 3 
Author information:
1Oral Pathology Department, Dental Faculty, Dental Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
2School of Medicine and Dentistry, Griffith University, Gold Coast, Q4222, Australia
3Pathology Department, Taleghani Hospital, Shahid Beheshti University of Medical Sciences Tehran, Iran
Abstract
Background: Lesions arising from odontogenic tissues cover a wide spectrum, from simple cysts to benign tumors and even carcinomas. Unicystic ameloblastoma (UA), multicystic ameloblastoma (MA), and ameloblastic carcinoma (AC) are relatively common among these odontogenic lesions. Nevertheless, the exact origin of these lesions remains unclear. It is noteworthy that BRCA1/2 mutations have been identified as potential contributing factors to the onset of various cancers. The aim of the current study was to scrutinize the BRCA1/2 expression profiles in UA, MA, and AC samples.
Methods: Biopsy specimens were collected from 60 patients, with 20 samples representing each lesion, sourced from the pathology department archive of a teaching hospital from 2000 to 2019. Immunohistochemical staining was conducted on all biopsy samples. Statistical analyses were performed using the Kruskal-Wallis H test. The differences in the data were compared with Mann- Whitney U test, and P<0.05 was considered to be significant.
Results: The Kruskal-Wallis test results revealed significant differences between the examined samples in terms of the cytoplasmic expression level of the BRCA1 basal cells (P<0.001). Regarding the cytoplasmic expression of BRCA1 stellate reticulum-like cells, there was a statistically significant difference between the examined groups (P<0.001). Furthermore, the results of the Kruskal-Wallis test demonstrated a significant difference concerning the cytoplasmic expression levels of BRCA2 basal cells between the studied groups (P<0.003). Based on the Kruskal-Wallis results, significant differences were found in terms of the cytoplasmic expression level of BRCA2 stellate reticulum-like cells between the investigated samples (P<0.001). The Mann-Whitney U test compared differences between the two examined groups.
Conclusion: The findings mainly revealed the expression of BRCA1/2 at the invasive front, which is composed of the most aggressive cells. Molecular interactions in this area may affect tumor progression. BRCA1/2 mutations can be a promising future treatment option for ameloblastoma and related lesions.
Keywords: Ameloblastoma, BRCA1, BRCA2, Immunohistochemistry, Jaw
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Irani S, Rafizadeh M. BRCA1/2 immunohistochemical analysis in unicystic ameloblastoma, multicystic ameloblastoma, and ameloblastic carcinoma. Avicenna J Dent Res. 2024; 16(3):140-145. doi:10.34172/ajdr.1776
Background
Lesions originating from odontogenic tissues encompass a diverse spectrum, ranging from simple cysts to benign tumors and even carcinomas (1). Unicystic ameloblastoma (UA) is characterized by slow growth and local aggressiveness, constituting 5%–15% of all ameloblastomas. Multicystic ameloblastoma (MA) is a benign yet locally aggressive odontogenic neoplasm with a high recurrence rate, comprising about 1–3% of all jaw tumors and cysts. Ameloblastic carcinoma (AC), the malignant variant of MA, is rare, constituting 2% of all odontogenic tumors. It may arise de novo or result from the malignant transformation of a long-standing MA, often with a history of multiple surgical procedures. AC carries a poor prognosis and a tendency to metastasize. Histologically, AC resembles MA but exhibits cytologic features of malignancy, such as cellular atypia and nuclear hyperchromatism (2). The etiology of MA is not entirely clear, but some studies suggest that mutations or molecular alterations play a significant role in its development; however, the precise sequence of events remains unknown.
BRCA1/2 genes, pivotal tumor suppressor genes, play a crucial role in DNA repair mechanisms. Mutations in BRCA1/2 are implicated in the onset of various cancers, including breast and ovarian cancers, with documented occurrences in 45–80% and 18–40% of breast and ovarian cancer patients, respectively (3,4). In prostate cancer, BRCA1/2 mutations are linked to more aggressive tumor types and an elevated risk of nodal and distant metastasis (5). Previous studies on oral squamous cell carcinoma and salivary gland tumors have demonstrated an increasedexpression level of BRCA1/2 in tissue samples (6-8).
The interaction between nuclear and BRCA1/2 proteins has been elucidated, showcasing the effectiveness of poly(ADP-ribose) polymerase inhibitors against tumors harboring BRCA1 and BRCA2 mutations (9). Currently, the predominant approach to ameloblastoma treatment is surgical resection, a method fraught with a considerable risk of recurrence (10).
Recognizing potential BRCA1/2 mutations associated with cases of ameloblastoma and related lesions could pave the way for novel treatment strategies. Immunohistochemistry, an accessible and cost-effective technique, is employed to determine the immunolocalization of BRCA1/2 proteins in various cancers, including salivary gland tumors, oral cancer, and ovarian cancer. Additionally, immunohistochemistry serves as a prognostic biomarker for these cancers (6). A deeper understanding of the implicated pathways holds the potential to unveil innovative therapeutic strategies in the management of these odontogenic lesions.
Materials and Methods
Samples
Biopsy specimens from 60 patients (20 samples for each lesion) were obtained from the archive of the Pathology Department of Taleghani Educational Hospital, Tehran, Iran, from 2000 to 2020. Hematoxylin and eosin (H&E) staining confirmed the previous diagnosis. The institutional review board approval number was 9703011051.
Immunohistochemical Staining
In summary, paraffin blocks were cut into 4 𝜇m thick sections. These sections were then subjected to deparaffinization and dehydration using graded alcohol. Antigen retrieval was performed in citrate buffer (pH = 6). To inhibit endogenous peroxidase activity, a Leica detection kit was utilized, followed by incubation with the primary mouse monoclonal anti-BRCA1 antibody (ab16780; Abcam, UK) at a 1:90 dilution and the primary rabbit polyclonal anti-BRCA2 antibody (ab216972; Abcam, UK) at a 1:80 dilution for 1 hour at room temperature.
Subsequent to primary antibody incubation, the sections were treated with a secondary antibody for 30 minutes. All sections were then immunostained with 3, 3’-diaminobenzidine for 5 minutes as a chromogen, with hematoxylin serving as a counterstain. Breast carcinoma tissue was utilized as a positive control. The primary antibody was omitted for negative control sections (11,12).
Detection and Scoring
Two independent pathologists reviewed the stained histological tissue sections. Cytoplasmic and nuclear staining were considered positive immunoreactivity for BRCA1/2. According to the manufacturer, immunopositivity was localized at the sites of DNA damage during double-strand breaks and recruitment to DNA damage. A semi-quantitative scoring system was used for the percentage of positively stained tumor cells, as follows:
Occasional staining rates of < 10%, 10%–40%, 40%–70%, and > 70% were considered negative, weak, moderate, and strong, respectively (8,13).
Statistical Analysis
The statistical analysis was performed using the SPSS static software package, version 20. The Kruskal-Wallis H test was used to determine if there was any statistically significant difference between the means of the groups. The differences in the means were compared with the Mann-Whitney U test, and a P value < 0.05 was considered to be significant.
Results
The Kruskal-Wallis test revealed significant differences between the examined samples in terms of the cytoplasmic and nuclear expression levels of the BRCA1 basal cells (P < 0.001 and P < 0.001, respectively). Regarding the cytoplasmic and nuclear expression levels of BRCA1 stellate reticulum-like cells, there were statistically significant differences between the studied groups (P < 0.001 and P < 0.001, respectively). Furthermore, the Kruskal-Wallis test results demonstrated significant differences concerning the cytoplasmic and nuclear expression levels of the BRCA2 basal cells between the intended groups (P < 0.003 and P < 0.005, respectively). Likewise, the results of the Kruskal-Wallis test showed significant differences in terms of the cytoplasmic and nuclear expression levels of BRCA2 stellate reticulum-like cells between the investigated samples (P < 0.001, P < 0.001, respectively). The Mann-Whitney U test compared differences between the two examined groups. The results of the BRCA1/2 staining in different types of lesions and cells are summarized in Tables 1 and 2. In addition, Figure 1 shows the subcellular localization of BRCA1/2 in different layers of the examined lesions.
Table 1.
The Expression Profile of BRCA1/2 in Unicystic Ameloblastoma, Multicystic Ameloblastoma, and Ameloblastic Carcinoma Using the Kruskal-Wallis Test
|
Variable
|
UA
|
MA
|
AC
|
P
value
|
| BRCA1 (cytoplasm of basal cells) |
0.001* |
| Mean ± SD |
18 ± 1 |
19 ± 1 |
11 ± 7 |
|
| Negative |
0 (0%) |
0 (0%) |
4 (20%) |
|
| Weak |
0 (0%) |
0 (0%) |
5 (25%) |
|
| Moderate |
3 (15%) |
3 (15%) |
5 (25%) |
|
| Strong |
17 (85%) |
17 (85%) |
6 (30%) |
|
| BRCA1 (nucleus of basal cells) |
|
|
|
0.001* |
| Mean ± SD |
19 ± 1 |
1 ± 3 |
0 ± 1 |
|
| Negative |
0 (0%) |
15 (75%) |
18 (90%) |
|
| Weak |
0 (0%) |
5 (25%) |
2 (10%) |
|
| Moderate |
2 (10%) |
0 (0%) |
0 (0%) |
|
| Strong |
18 (85%) |
0 (0%) |
0 (0%) |
|
| BRCA1 (cytoplasm of stellate reticulum-like cells) |
0.015* |
| Mean ± SD |
18 ±2 |
16 ± 2 |
16 ± 2 |
|
| Negative |
0 (0%) |
0 (0%) |
0 (0%) |
|
| Weak |
0 (0%) |
0 (0%) |
0 (0%) |
|
| Moderate |
4 (20%) |
9 (45%) |
13 (65%) |
|
| Strong |
16 (80%) |
11 (55%) |
7 (35%) |
|
| BRCA1 (nucleus of stellate reticulum-like cells) |
0.001* |
| Mean ± SD |
19 ± 1 |
2 ± 3 |
12 ± 8 |
|
| Negative |
0 |
14 (70%) |
5 (25%) |
|
| Weak |
0 |
6 (30%) |
0 |
|
| Moderate |
2 (10%) |
0 (0%) |
7 (35%) |
|
| Strong |
18 (90%) |
0 (0%) |
8 (40%) |
|
| BRCA2 (cytoplasm of basal cells) |
0.005 * |
| Mean ± SD |
6 ± 7 |
11 ± 6 |
3 ± 3 |
|
| Negative |
7 (35%) |
4 (20%) |
8 (40%) |
|
| Weak |
7 (35%) |
5 (25%) |
12 (60%) |
|
| Moderate |
3 (15%) |
7 (35%) |
0 (0%) |
|
| Strong |
3 (15%) |
4 (20%) |
0 (0%) |
|
| BRCA2 (nucleus of basal cells) |
|
|
|
0.001* |
| Mean ± SD |
8 ± 7 |
3 ± 5 |
1 ± 2 |
|
| Negative |
7 (35%) |
14 (70%) |
15 (75%) |
|
| Weak |
5 (25%) |
2 (10%) |
5 (25%) |
|
| Moderate |
3 (15%) |
4 (20%) |
0 (0%) |
|
| Strong |
5 (25%) |
0 (0%) |
0 (0%) |
|
| BRCA2 (cytoplasm of stellate reticulum-like cells) |
0.001 * |
| Mean ± SD |
18 ± 2 |
9 ± 5 |
8 ± 7 |
|
| Negative |
0 (0%) |
0 (0%) |
6 (5%) |
|
| Weak |
0 (0%) |
14 (70%) |
7 (45%) |
|
| Moderate |
5 (30%) |
3 (15%) |
3 (15%) |
|
| Strong |
15 (70%) |
3 (15%) |
4 (20%) |
|
| BRCA2 (nucleus of stellate reticulum-like cells) |
0.001 * |
| Mean ± SD |
13 ± 2 |
6 ± 5 |
5 ± 7 |
|
| Negative |
0 (0%) |
6 (30%) |
11 (55%) |
|
| Weak |
4 (20%) |
8 (40%) |
4 (20%) |
|
| Moderate |
16 (80%) |
6 (30%) |
3 (15%) |
|
| Strong |
0 (0%) |
0 (0%) |
2 (10%) |
|
Note. UA: Unicystic ameloblastoma; MA: Multicystic ameloblastoma; AC: Ameloblastic carcinoma; SD: Standard devation. *P < 0.05.
Table 2.
The Comparison of Means Between Two Groups Using Mann-Whitney U Test
|
Type of Antibody and Subcellular Localization
|
UA and MA
|
UA and AC
|
MA and AC
|
| BRCA1 (cytoplasm of basal cells) |
P < 0.001 |
P < 0.001 |
P < 0.001 |
| BRCA1(nucleus of basal cells) |
P < 0.001 |
P < 0.001 |
P < 0.001 |
| BRCA1 (cytoplasm of stellate reticulum-like cells) |
P < 0.001 |
P < 0.001 |
P < 0.001 |
| BRCA1 (nucleus of stellate reticulum-like cells) |
P < 0.001 |
P < 0.001 |
P < 0.001 |
| BRCA2 (cytoplasm of basal cells) |
P < 0.032 |
P < 0.001 |
P < 0.001 |
| BRCA2 (nucleus of basal cells) |
P < 0.033 |
P < 0.002 |
P < 0.001 |
| BRCA2 (cytoplasm of stellate reticulum-like cells) |
P < 0.001 |
P < 0.001 |
P < 0.001 |
| BRCA2 (nucleus of stellate reticulum-like cells) |
P < 0.001 |
P < 0.001 |
P < 0.001 |
Note. UA: Unicystic ameloblastoma; MA: Multicystic ameloblastoma; AC: Ameloblastic carcinoma.
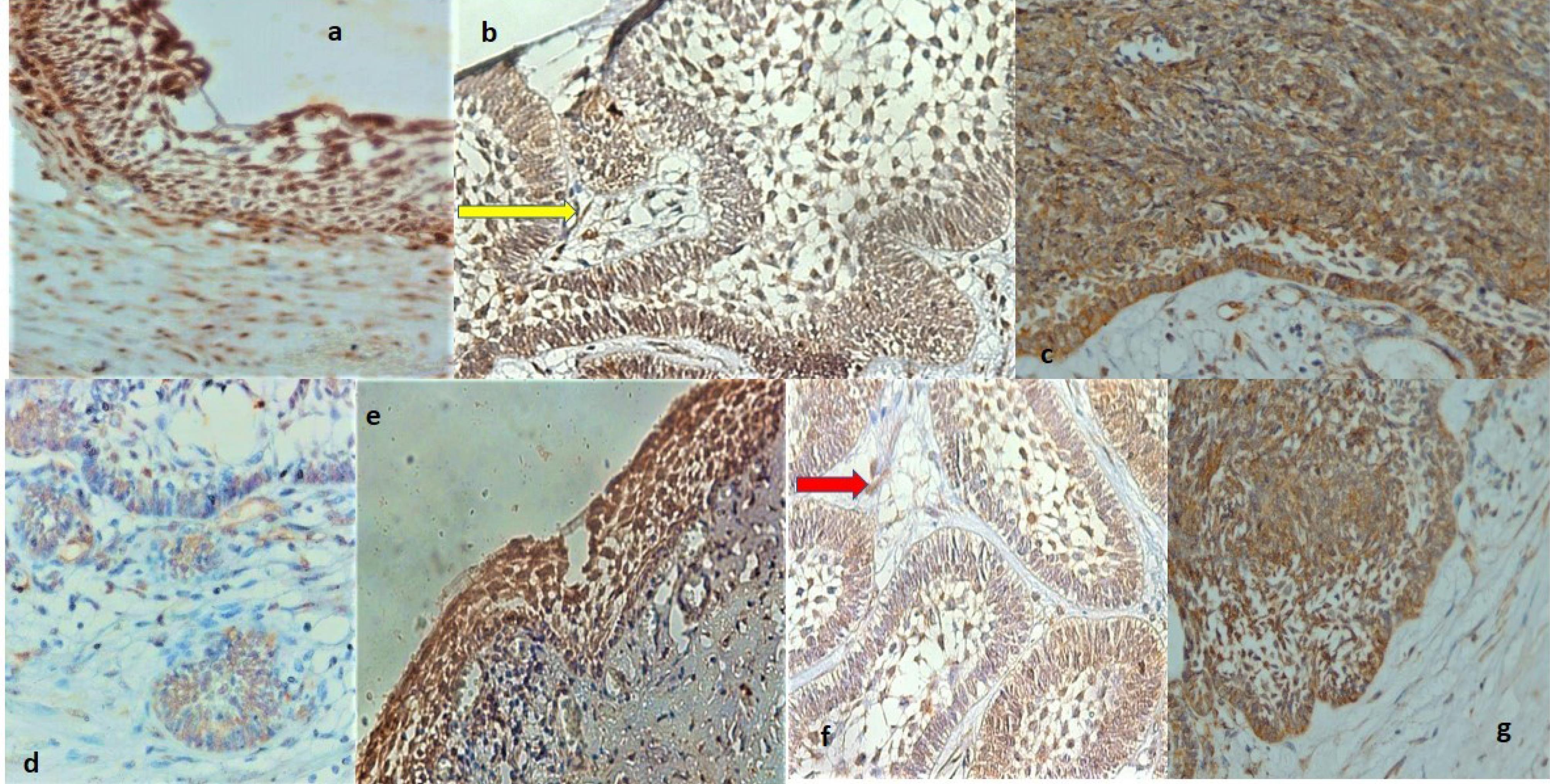

Figure 1.
(a) High magnification showing strong BRCA1 positivity in unicystic ameloblastoma, (b) Paraffin section of multicystic ameloblastoma illustrating strong cytoplasmic and nuclear positivity for BRCA1 in the basal layer and stellate reticulum-like cells, (c) High power section of ameloblastic carcinoma demonstrating strong positive products of BRCA1 localized in the cytoplasm and nucleus of ameloblastic epithelium ( × 400), (d) Histologic section of multicystic ameloblastoma displaying cytoplasmic and nuclear BRCA1 labeling in the small islands and stromal cells, (e) High-power magnification of unicystic ameloblastoma exhibiting strong cytoplasmic and nuclear BRCA2 immunoreactivity, (f) The high-power magnification view of multicystic ameloblastoma showing strong immunolocalization of BRCA2 in the cytoplasm of basal layer cells and stellate reticulum-like cells, and (g) High magnification of ameloblastic carcinoma indicating strong BRCA2 immunoreactivity in the cytoplasm and nuclei of stellate-reticulum-like cells. Note. (a) Cytoplasmic and nuclear staining are evident in the cystic epithelial lining and stromal cells in the cyst wall ( × 400). (b) The yellow arrow indicates the BRCA1 positivity in stromal cells ( × 400). (d) Note that endothelial cells also demonstrate BRCA1 immunopositivity ( × 400). (e) A few stromal cells are stained as well ( × 400). (f) Some nuclei also exhibit BRCA2 immunostaining. The red arrow represents BRCA2 positivity in stromal cells ( × 400). (g) Strong cytoplasmic and nuclear staining of basal layer cells are also evident ( × 400).
.
(a) High magnification showing strong BRCA1 positivity in unicystic ameloblastoma, (b) Paraffin section of multicystic ameloblastoma illustrating strong cytoplasmic and nuclear positivity for BRCA1 in the basal layer and stellate reticulum-like cells, (c) High power section of ameloblastic carcinoma demonstrating strong positive products of BRCA1 localized in the cytoplasm and nucleus of ameloblastic epithelium ( × 400), (d) Histologic section of multicystic ameloblastoma displaying cytoplasmic and nuclear BRCA1 labeling in the small islands and stromal cells, (e) High-power magnification of unicystic ameloblastoma exhibiting strong cytoplasmic and nuclear BRCA2 immunoreactivity, (f) The high-power magnification view of multicystic ameloblastoma showing strong immunolocalization of BRCA2 in the cytoplasm of basal layer cells and stellate reticulum-like cells, and (g) High magnification of ameloblastic carcinoma indicating strong BRCA2 immunoreactivity in the cytoplasm and nuclei of stellate-reticulum-like cells. Note. (a) Cytoplasmic and nuclear staining are evident in the cystic epithelial lining and stromal cells in the cyst wall ( × 400). (b) The yellow arrow indicates the BRCA1 positivity in stromal cells ( × 400). (d) Note that endothelial cells also demonstrate BRCA1 immunopositivity ( × 400). (e) A few stromal cells are stained as well ( × 400). (f) Some nuclei also exhibit BRCA2 immunostaining. The red arrow represents BRCA2 positivity in stromal cells ( × 400). (g) Strong cytoplasmic and nuclear staining of basal layer cells are also evident ( × 400).
Discussion
BRCA1/2 mutations, known for their role in certain cancers, present an intriguing avenue of exploration. For example, women carrying BRCA1/2 mutations face a heightened risk of breast and ovarian cancers (14). In addition, germline mutations in BRCA1 and BRCA2 have been observed in familial pancreatic ductal adenocarcinoma (15). A prior study has indicated that BRCA1 mutations seem to be more frequent than BRCA2 mutations (7).
Ameloblastoma emerges as the most common benign odontogenic tumor, notorious for its elevated recurrence rate (reaching up to 50%) and a variable but present risk of malignant transformation. Unraveling the molecular events and implicated genes in MA has the potential to mitigate recurrence rates and decrease the likelihood of malignant transformations (16).
In the current study, strong immunoreactivity for BRCA1/2 was mainly found in UAs compared to MAs and ACs (Table 1). These results may indicate that DNA damage is involved in the development of UA and may contribute to tumor progression and malignant transformation. It was previously proposed that DNA damage is involved in the development of MAs (17). Of importance, BRCA1 and BRCA2 modulate the activity of some transcription regulators, such as p53 and Smad3 (18). A high expression level of p53 and Smad3 has been investigated in MAs (19,20). It has been proposed that loss or inactivation of tumor suppressor genes contributes to acquiring neoplastic growth and more aggressive types of tumors (21).
Different genes are involved in the development of odontogenic lesions. Some genetic and molecular factors play a critical role in the etiopathogenesis of ameloblastoma. These factors, including sonic hedgehog and WNT/β-catenin signaling pathways, promote the transformation of the odontogenic epithelium into ameloblastoma. It is hypothesized that the development of ameloblastoma is linked to the enamel organ, remnants of the odontogenic epithelium, and the lining of the odontogenic cysts (22). This hypothesis is supported by the similar expression profiles of cytokeratin and vimentin in the developing tooth germ and ameloblastoma (23). In addition, molecular biological studies have demonstrated that gene mutations play an important role in the pathogenesis of ameloblastoma (24-26). For example, the BRAF V600E mutation has been reported in 83% of UAs, 82% of Mas, and 38% of ACs (27). Further, in a study on 84 cases of mandibular ameloblastoma, BRAF V600E positivity was documented in 78.6% of cases. The authors suggested BRAF inhibitors as potential therapeutic strategies for the treatment of ameloblastoma (28). A previously published paper has indicated higher rates of allelic loss of L- myc and pten genes in UA samples compared to MAs and ACs (29). The smoothened mutation is also common in ameloblastoma (30). A germline mutation of the PTCH gene has also been reported in UA and MA samples (31).
Epithelial–mesenchymal transition (EMT) has an essential role in tumor cell migration and invasion. Tumor cells undergoing EMT can acquire stem cell-like features. The BRCA1 mutation activates the EMT phenomenon and induces the dedifferentiation of stem cells in mammary tumor cells; therefore, it promotes cancer cell migration and invasion (32). The BRCA1 mutation, known for its influence on the EMT phenomenon, induces the dedifferentiation of stem cells in mammary tumor cells, thereby promoting cancer cell migration and invasion. This phenomenon has also been investigated in the context of ameloblastoma, revealing the potential role of EMT in tumor growth and bone destruction (33). A prior study has shown that loss of BRCA1 in breast epithelial cells may affect the tumor microenvironment, which successively promotes tumor growth and the metastatic potential of BRCA1-deficient tumor cells (34) The contribution of stromal cells, including fibroblasts, in the pathogenesis of ameloblastoma by forming a specific microenvironment has been reported in previous research (24). In addition, a published research paper has demonstrated widespread expression of dental epithelial stem cell markers in ameloblastoma samples. The researchers concluded that these cells could be cancer stem cells (35). A former study indicated higher expression levels of NOTCH2 and NOTCH3 at the interface between the ameloblastoma epithelium and adjacent stroma, which may indicate the acquisition of an invasive phenotype (36). Detailed research has shown immunopositivity for cancer stem cell markers and Ki-67, a cell proliferation marker, in the peripheral columnar epithelium of MAs. The location of these peripheral cells at the invasive front suggests their role in maintaining tumor growth. Interestingly, Ki67 expression in MAs was primarily in basal and supra-basal cells, while in ACs, it was observed in basal and central cells, with a higher expression level in ACs compared to MAs (37). In the present study, BRCA1/2 immunoreactivity was identified in various cells, including peripheral columnar cells (invasive front), stellate reticulum-like cells, stromal cells such as fibroblasts across all samples, especially at the interface, and endothelial cells (Figure 1). These findings suggest a potential association between BRCA1/2 mutations, EMT activity, activation of cancer stem cells, and stromal involvement in the development of cystic lesions, tumor progress, and malignant transformation. Consequently, BRCA1/2 mutations heighten the risk of invasiveness in tumor cells.
Conclusion
In recent times, several studies have significantly enhanced our comprehension of the molecular pathogenesis underlying ameloblastoma. The majority of cases exhibit mutations affecting various genes, and the identification of these mutations has the potential to predict the biological behavior of ameloblastoma. Notably, certain mutations, such as the BRAF V600E mutation, can serve as valuable diagnostic markers. In the present study, a higher expression level of BRCA1/2 was observed at the invasive front, where the most aggressive cells are concentrated. These findings may suggest that peripheral and stromal cells could serve as potential prognostic factors for tumor aggressiveness. Moreover, BRCA1/2 mutations present a promising avenue for prospective treatment options for ameloblastoma and related lesions. The identification of patients with BRCA1/2 mutations becomes paramount, highlighting a crucial aspect of treatment planning with substantial implications for therapeutic strategies. More investigation with more samples is required to study the role of the BRCA1/2 mutation in the development of ameloblastoma and malignant transformation. In addition, more details should be understood about the pathways involved in odontogenic lesion development.
Acknowledgements
The authors would like to express their deepest gratitude to Hamadan University of Medical Sciences for the technical and financial support for this study.
Author’s Contribution
Conceptualization: Soussan Irani.
Data curation: Soussan Irani, Mitra Rafizadeh.
Formal analysis: Soussan Irani.
Funding acquisition: Soussan Irani.
Investigation: Soussan Irani, Mitra Rafizadeh.
Methodology: Soussan Irani.
Supervision: Soussan Irani.
Writing–original draft: Soussan Irani.
Writing–review & editing: Soussan Irani, Mitra Rafizadeh.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The institutional review board approval number was 9703011051.
References
- Irani S, Foroughi F. Histologic variants of calcifying odontogenic cyst: a study of 52 cases. J Contemp Dent Pract 2017; 18(8):688-94. doi: 10.5005/jp-journals-10024-2108 [Crossref] [ Google Scholar]
- Masthan KM, Anitha N, Krupaa J, Manikkam S. Ameloblastoma. J Pharm Bioallied Sci 2015; 7(Suppl 1):S167-70. doi: 10.4103/0975-7406.155891 [Crossref] [ Google Scholar]
- Kitami K, Kitami M, Kaku M, Wang B, Komatsu Y. BRCA1 and BRCA2 tumor suppressors in neural crest cells are essential for craniofacial bone development. PLoS Genet 2018; 14(5):e1007340. doi: 10.1371/journal.pgen.1007340 [Crossref] [ Google Scholar]
- Mehrgou A, Akouchekian M. The importance of BRCA1 and BRCA2 genes mutations in breast cancer development. Med J Islam Repub Iran 2016; 30:369. [ Google Scholar]
- Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013; 31(14):1748-57. doi: 10.1200/jco.2012.43.1882 [Crossref] [ Google Scholar]
- Irani S, Rafizadeh M. BRCA1/2 expression patterns in different grades of oral squamous cell carcinoma. Middle East J Cancer 2020; 11(4):390-8. doi: 10.30476/mejc.2020.81282.0 [Crossref] [ Google Scholar]
- Stoppa-Lyonnet D. The biological effects and clinical implications of BRCA mutations: where do we go from here?. Eur J Hum Genet 2016; 24(Suppl 1):S3-9. doi: 10.1038/ejhg.2016.93 [Crossref] [ Google Scholar]
- Irani S, Bidari-Zerehpoush F. BRCA1/2 mutations in salivary pleomorphic adenoma and carcinoma-ex-pleomorphic adenoma. J Int Soc Prev Community Dent 2017; 7(Suppl 3):S155-62. doi: 10.4103/jispcd.JISPCD_184_17 [Crossref] [ Google Scholar]
- Patel M, Nowsheen S, Maraboyina S, Xia F. The role of poly(ADP-ribose) polymerase inhibitors in the treatment of cancer and methods to overcome resistance: a review. Cell Biosci 2020; 10:35. doi: 10.1186/s13578-020-00390-7 [Crossref] [ Google Scholar]
- Cadavid AM, Araujo JP, Coutinho-Camillo CM, Bologna S, Junior CA, Lourenço SV. Ameloblastomas: current aspects of the new WHO classification in an analysis of 136 cases. Surg Exp Pathol 2019; 2(1):17. doi: 10.1186/s42047-019-0041-z [Crossref] [ Google Scholar]
- Irani S, Jafari B. Expression of vimentin and CD44 in mucoepidermoid carcinoma: a role in tumor growth. Indian J Dent Res 2018; 29(3):333-40. doi: 10.4103/ijdr.IJDR_184_17 [Crossref] [ Google Scholar]
- Irani S, Dehghan A. Expression of vascular endothelial-cadherin in mucoepidermoid carcinoma: role in cancer development. J Int Soc Prev Community Dent 2017; 7(6):301-7. doi: 10.4103/jispcd.JISPCD_323_17 [Crossref] [ Google Scholar]
- Irani S, Dehghan A. The expression and functional significance of vascular endothelial-cadherin, CD44, and vimentin in oral squamous cell carcinoma. J Int Soc Prev Community Dent 2018; 8(2):110-7. doi: 10.4103/jispcd.JISPCD_408_17 [Crossref] [ Google Scholar]
- Kotsopoulos J. BRCA mutations and breast cancer prevention. Cancers (Basel) 2018; 10(12):524. doi: 10.3390/cancers10120524 [Crossref] [ Google Scholar]
- Salo-Mullen EE, O’Reilly EM, Kelsen DP, Ashraf AM, Lowery MA, Yu KH. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer 2015; 121(24):4382-8. doi: 10.1002/cncr.29664 [Crossref] [ Google Scholar]
- Ghai S. Ameloblastoma: an updated narrative review of an enigmatic tumor. Cureus 2022; 14(8):e27734. doi: 10.7759/cureus.27734 [Crossref] [ Google Scholar]
- Toprani SM. DNA damage and repair scenario in ameloblastoma. Oral Oncol 2020; 108:104804. doi: 10.1016/j.oraloncology.2020.104804 [Crossref] [ Google Scholar]
- Gorodetska I, Kozeretska I, Dubrovska A. BRCA genes: the role in genome stability, cancer stemness and therapy resistance. J Cancer 2019; 10(9):2109-27. doi: 10.7150/jca.30410 [Crossref] [ Google Scholar]
- Salehinejad J, Zare-Mahmoodabadi R, Saghafi S, Jafarian AH, Ghazi N, Rajaei AR. Immunohistochemical detection of p53 and PCNA in ameloblastoma and adenomatoid odontogenic tumor. J Oral Sci 2011; 53(2):213-7. doi: 10.2334/josnusd.53.213 [Crossref] [ Google Scholar]
- Karathanasi V, Tosios KI, Nikitakis NG, Piperi E, Koutlas I, Trimis G. TGF-β1, Smad-2/-3, Smad-1/-5/-8, and Smad-4 signaling factors are expressed in ameloblastomas, adenomatoid odontogenic tumors, and calcifying cystic odontogenic tumors: an immunohistochemical study. J Oral Pathol Med 2013; 42(5):415-23. doi: 10.1111/jop.12016 [Crossref] [ Google Scholar]
- Borkosky SS, Gunduz M, Beder L, Tsujigiwa H, Tamamura R, Gunduz E. Allelic loss of the ING gene family loci is a frequent event in ameloblastoma. Oncol Res 2010; 18(10):509-18. doi: 10.3727/096504010x12704916124864 [Crossref] [ Google Scholar]
- Effiom OA, Ogundana OM, Akinshipo AO, Akintoye SO. Ameloblastoma: current etiopathological concepts and management. Oral Dis 2018; 24(3):307-16. doi: 10.1111/odi.12646 [Crossref] [ Google Scholar]
- Sherlin HJ, Natesan A, Ram P, Ramani P, Thiruvenkadam C. Immunohistochemical profiling of ameloblastomas using cytokeratin, vimentin, smooth muscle actin, CD34 and S100. Ann Maxillofac Surg 2013; 3(1):51-7. doi: 10.4103/2231-0746.110084 [Crossref] [ Google Scholar]
- Fuchigami T, Ono Y, Kishida S, Nakamura N. Molecular biological findings of ameloblastoma. Jpn Dent Sci Rev 2021; 57:27-32. doi: 10.1016/j.jdsr.2020.12.003 [Crossref] [ Google Scholar]
- Farshbaf A, Zare R, Mohajertehran F, Mohtasham N. New diagnostic molecular markers and biomarkers in odontogenic tumors. Mol Biol Rep 2021; 48(4):3617-28. doi: 10.1007/s11033-021-06286-0 [Crossref] [ Google Scholar]
- Irani S, Mohsenifar Z. Endocan, ET-1, and ETAR expression profiles in unicystic ameloblastoma, multicystic ameloblastoma, and ameloblastic carcinoma. Middle East J Cancer 2019; 10(3):167-74. doi: 10.30476/mejc.2019.78562 [Crossref] [ Google Scholar]
- Diniz MG, Gomes CC, Guimarães BV, Castro WH, Lacerda JC, Cardoso SV. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumour Biol 2015; 36(7):5649-53. doi: 10.1007/s13277-015-3238-0 [Crossref] [ Google Scholar]
- do Canto AM, da Silva Marcelino BM, Schussel JL, Wastner BF, Sassi LM, Corrêa L. Immunohistochemical analysis of BRAF V600E mutation in ameloblastomas. Clin Oral Investig 2019; 23(2):779-84. doi: 10.1007/s00784-018-2494-y [Crossref] [ Google Scholar]
- Nodit L, Barnes L, Childers E, Finkelstein S, Swalsky P, Hunt J. Allelic loss of tumor suppressor genes in ameloblastic tumors. Mod Pathol 2004; 17(9):1062-7. doi: 10.1038/modpathol.3800147 [Crossref] [ Google Scholar]
- Sweeney RT, McClary AC, Myers BR, Biscocho J, Neahring L, Kwei KA. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet 2014; 46(7):722-5. doi: 10.1038/ng.2986 [Crossref] [ Google Scholar]
- Farias LC, Gomes CC, Brito JA, Galvão CF, Diniz MG, de Castro WH. Loss of heterozygosity of the PTCH gene in ameloblastoma. Hum Pathol 2012; 43(8):1229-33. doi: 10.1016/j.humpath.2011.08.026 [Crossref] [ Google Scholar]
- Sengodan SK, K HS, Nadhan R, Srinivas P. Regulation of epithelial to mesenchymal transition by BRCA1 in breast cancer. Crit Rev Oncol Hematol 2018; 123:74-82. doi: 10.1016/j.critrevonc.2018.01.008 [Crossref] [ Google Scholar]
- Siar CH, Ng KH. Epithelial-to-mesenchymal transition in ameloblastoma: focus on morphologically evident mesenchymal phenotypic transition. Pathology 2019; 51(5):494-501. doi: 10.1016/j.pathol.2019.04.004 [Crossref] [ Google Scholar]
- Miklikova S, Trnkova L, Plava J, Bohac M, Kuniakova M, Cihova M. The role of BRCA1/2-mutated tumor microenvironment in breast cancer. Cancers (Basel) 2021; 13(3):575. doi: 10.3390/cancers13030575 [Crossref] [ Google Scholar]
- Pagella P, Catón J, Meisel CT, Mitsiadis TA. Ameloblastomas exhibit stem cell potential, possess neurotrophic properties, and establish connections with trigeminal neurons. Cells 2020; 9(3):644. doi: 10.3390/cells9030644 [Crossref] [ Google Scholar]
- Jackstadt R, van Hooff SR, Leach JD, Cortes-Lavaud X, Lohuis JO, Ridgway RA, et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell 2019;36(3):319-36.e7. 10.1016/j.ccell.2019.08.003.
- Martínez-Martínez M, Mosqueda-Taylor A, Carlos-Bregni R, Pires FR, Delgado-Azanero W, Neves-Silva R. Comparative histological and immunohistochemical study of ameloblastomas and ameloblastic carcinomas. Med Oral Patol Oral Cir Bucal 2017; 22(3):e324-32. doi: 10.4317/medoral.21901 [Crossref] [ Google Scholar]