Avicenna J Dent Res. 16(3):177-183.
doi: 10.34172/ajdr.1766
Original Article
Developing a Prediction Model for Periodontal Disease Using Ordinal Random Forest
Maryam Farhadian 1, *  , Zahra Torkashvand 2, Parisa Shokouhi 3, Parviz Torkzaban 4
, Zahra Torkashvand 2, Parisa Shokouhi 3, Parviz Torkzaban 4
Author information:
1Department of Biostatistics, School of Public Health and Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Biostatistics, School of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran
3Dental School, Hamadan University of Medical Sciences, Hamadan, Iran
4Dental Research Center, Department of Periodontics, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Background: Periodontitis, a common inflammatory disease affecting oral health, is a major public health concern. Early detection of this disease is highly important in order to achieve less invasive and less costly treatment. The ordinal nature of different levels of periodontitis is usually not considered in many studies. It is often recognized and used as a nominal variable, in which case some information related to the ordinal nature is ignored. The aim of this study was to use continuation ratio logistic and ordinal random forest (RF) models to predict the three stages of periodontitis.
Methods: Overall, this study evaluated 300 patients referred to the Periodontology Department of Hamadan University, western Iran, between September 2016 and June 2018. The performance of continuous ratio logistic and ordinal forest models was evaluated using the same set of training and test data to predict different types of periodontitis (gingivitis, localized, and generalized periodontitis) based on input variables. Accuracy, kappa, gamma, Somers’d, and precision were utilized for comparison.
Results: The results confirmed the higher predictive ability of the ordinal RF model compared to the logistic continuation ratio model for all scoring indices (accuracy of 0.87 vs. 0.80). Alveolar bone loss, attachment loss, probing pocket depth, simplified oral hygiene index, and plaque index were identified as the most important variables.
Conclusion: Due to the ordinal nature of different levels of periodontal disease, the use of accurate prediction models such as ordinal RF is suggested since they can take into account the ordinal nature of the response in predicting and evaluating the effect of important variables. Ordinal RF is a well-suited machine learning technique for developing accurate predictive models of periodontal disease risk.
Keywords: Ordinal response, Classification, Periodontitis, Ordinal random forest, Continuation-ratio logistic model
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Farhadian M, Torkashvand Z, Shokouhi P, Torkzaban P. Developing a prediction model for periodontal disease using ordinal random forest. Avicenna J Dent Res. 2024; 16(3):177-183. doi:10.34172/ajdr.1766
Background
Periodontitis, a common inflammatory disease affecting oral health, is a major public health concern. Periodontal diseases are common and lead to physical, functional, and biological complications, as well as affecting the economic, social, and psychological dimensions of patients’ lives. Periodontitis is a relatively silent disease that, if left untreated in its early stages, can lead to relatively irreversible damage to the supporting structures of the teeth, including the gingiva, periodontal ligament, and alveolar bone, and eventually to tooth loss (1-4). The global prevalence of periodontitis is estimated to be 20%–50%. In Iran, the prevalence of periodontal disease has also been reported to be 20%–50%. The prevalence of gingivitis and periodontitis in patients between the ages of 35 and 70 years was 58.5% and 41.5%, respectively, in a study conducted in Rafsanjan, Iran (5).
Periodontitis is a chronic inflammatory disease of the gums that progresses through several stages. In the first stage, the plaque and gums are affected by an infection called gingivitis, which is an inflammation of the gums. The next stage involves the formation of plaque-filled pockets, followed by further loss of the tooth’s supporting tissues, leading to deeper pockets. Eventually, the tissue is destroyed, and the teeth may be lost. Symptoms of periodontal disease, such as bleeding gums, tooth shifting, and loss of the front tooth papilla, can affect a person’s ability to eat, speak, socialize, and perform various daily activities (6-10).
The role of periodontal disease in several other serious health problems, such as cardiovascular disease, kidney disease, diabetes, endometriosis, and oral cancer, has also been reported. In recent years, more attention has been paid to the impact of periodontal disease on people’s quality of life. Therefore, early detection of this disease is highly important to enable less invasive and cost-effective treatment (11-14).
Periodontal disease is a progressive condition that progresses through several stages, each characterized by increasing severity of symptoms and damage to the supporting structures of the teeth. Understanding these stages is essential for effective prevention and treatment. Early detection and treatment can effectively reverse gingivitis and manage more advanced stages of the disease (15).
As in all fields of medicine, successful treatment in dentistry is only possible with a correct diagnosis of the disease. A conventional diagnosis is made by a simultaneous clinical examination, X-ray, and objective evaluation of the patient’s medical and dental history. The dentist uses the diagnosis as the basis for a treatment plan. Emphasis on staging and grading helps clarify the extent and complexity of the patient’s periodontal condition, thereby influencing treatment decisions and prognostic expectations (16-18).
However, these examinations may not be sufficient, especially for inexperienced dentists, to make a correct diagnosis. Therefore, intelligent machine learning methods, such as artificial neural networks, support vector machines (SVMs), and random forests (RFs), can be applied in the development of decision support systems, as this capability has brought about a great change in healthcare. The development of such support systems can help dentists diagnose diseases and then organize effective treatment (16,17).
Predictive models have shown significant potential in the early detection of periodontal disease. There is a wide range of models for assessing the relationships between dependent and independent variables and predicting responses. Model selection is often achieved by measuring the scale of the response variable. In many medical and dental research studies, the response variable to be predicted or compared is ordinal in nature. Some examples are toxicity levels of substances rated as mild, moderate, or severe (18-20).
Although several studies have used machine learning techniques to build risk prediction models for periodontal disease, most of them have ignored the sequential nature of the different stages of periodontitis (16,21). Ordinal RF is an ensemble learning method that can effectively handle ordinal outcome variables, which is useful for modelling the progression of periodontal disease severity. It also provides insight into the relative importance of different risk factors (22).
Due to the ordinal nature of the different stages of periodontitis, this study aimed to use the continuation ratio logistic and ordinal RF models to predict the stages of periodontitis and then compare the performance of these models using the same training and test data sets.
Materials and Methods
The records of 300 patients (age range of the patients: 18-67 years) referred to the Periodontology Department of Hamadan University of Medical Sciences, Western Iran, between September 2016 and June 2018 were reviewed in this cross-sectional study. Variables were selected based on consultations with two experienced periodontists. The values of the desired variables for each patient were obtained from his file and recorded according to the principles of confidentiality. The periodontal examination of the patients included assessment of the periodontal condition and determination of pocket depth and plaque, assessment of clinical adhesion loss, and evaluation of the presence or absence of bleeding on probing.
Continuation Ratio Logistic Model
Assume that the response vector yi belongs to one ordinal class k, where k = 1,…, K and i = 1,…, n (n: number of individuals). In addition, xi represents a covariate vector of size p. A continuation ratio regression model, like binary logistic regression, models the logit rather than the probability modeling:
Therefore, the likelihood for the continuation ratio model is the product of the conditional independent binomial terms as follows:
is a vector of parameter. A model including K − 1 different β vectors may be overparameterized. To simplify, one commonly fits a constrained continuation model, which includes the K−1 thresholds (α2.…. αK) and one common set of p slope parameters slope parameters (β1.….βp). To fit a continuation ratio model, the original dataset can be restructured by forming K - 1 subsets, where for classes k = 2,..., K, the subset contains those observations in the original dataset up to class k. In addition, for the k-th subset, the outcome is dichotomized as y = 1 if the ordinal class is k and y = 0 otherwise. Further, an indicator is constructed for each subset, representing subset membership. The K−1 subsets are then added together to form the restructured dataset, which implies the K−1 conditionally independent datasets. Applying a logistic regression model to this restructured dataset yields a continuation ratio model(23,24).
The continuation ratio model is a type of ordinal logistic regression that focuses on the probability of being in a certain category or higher of an ordinal outcome.
Ordinal Forest Model
The ordinal forest model is an RF-based predictive classifier for ordinal response variables. RF, or random decision forest, is a supervised learning algorithm based on ensemble methods. In this algorithm, a collection of decision trees (DT) consisting of a group of trees is formed randomly. The method of forming a collection of trees is often called bagging. The main idea of bagging is that using a combination of learning models increases the overall accuracy of the model. Simply put, by randomly bagging multiple trees, decisions are made and combined to produce more accurate and stable predictions. The RF algorithm does not use all the sample observations to build the tree but selects a random sample by replacing the observations (bootstrap sampling). The selected observations are called experimental samples, and the remaining observations are called out-of-bag samples. DTs are constructed using learning observations, and out-of-bag samples are used to measure the impurity of the tree. The ordinal forest also calculates the variable importance measure, which describes which features are more relevant to the ordinal response (22,25-26).
Variables such as age, gender, smoking, attachment loss (AL), plaque index [PI (%)], probing pocket depth (PPD), gingival index (GI), alveolar bone loss (ABL; score: 0 for ABL < 20%, 1 for 20 < ABL < 50%, 2 for ABL > 50% based on radiographs), papillary bleeding index (PBI), mobility (MB), and simplified oral hygiene index were used as input (or independent) and different levels of severity of periodontal disease were considered as an ordinal output variable (or dependent) consisting of three classes (gingivitis, localized periodontitis, and generalized periodontitis).
From the information of 300 patients in this dataset, 200 people were randomly selected for the training dataset, and 100 people (including 55, 22, and 28 patients with gingivitis, localized periodontitis, and generalized periodontitis, respectively) were included in the test dataset.
To predict different levels of periodontitis based on the input variables, the performance of continuation ratio logistic and ordinal RF models was evaluated using the same set of training and test data. The models (continuation ratio logistic and ordinal RF) were trained and tested using 70% and 30% of the training and test data, respectively. The kappa statistic, gamma statistic, Somers’d, and accuracy were used for comparison. It should be noted that the gamma statistic and Somers’d are measures that take into account the ordinal nature of the predictions.
Results
The distribution of the study variables in the three stages of periodontitis is provided in Table 1. The values of several variables increase with an increase in the severity of the disease.
Table 1.
Comparison of the Distribution of Various Variables Between Three Classes (Stages) of Periodontal Disease
|
Variable
|
Gingivitis
|
Localized Periodontitis
|
Generalized Periodontitis
|
| Age, Mean (SD ) |
32.91 (10.77) |
33.88 (10.52) |
34.23 (12.38) |
| Attachment loss, Mean (SD ) |
1.63 (0.47) |
2.24 (0.61) |
3.39 (0.81) |
| Plaque index (%), Mean (SD ) |
43.46 (17.82) |
62.13 (17.80) |
74.55 (15.09) |
| Probing pocket depth, Mean (SD ) |
1.48 (0.32) |
2.01 (0.54) |
3.25 (0.78) |
| Gender, No. (%) |
Female |
65 (40.6) |
19 (31.7) |
29 (36.3) |
| Male |
95 (59.4) |
41 (68.3) |
51 (63.8) |
| Smoking, No. (%) |
No |
148 (92.5) |
54 (90) |
65 (81.2) |
| Yes |
12 (7.5) |
6 (10) |
15 (18.8) |
| Gingival index, No. (%) |
Grade I |
48 (30) |
6 (10) |
5 (15) |
| Grade II |
78 (48.8) |
24 (40) |
27 (33.8) |
| Grade III |
31 (19.4) |
26 (43.3) |
39 (43.8) |
| Grade IV |
3 (1.9) |
4 (6.7) |
9 (7.6) |
| Alveolar bone loss, No. (%) |
0 |
153 (95.6) |
7 (11.7) |
2 (2.5) |
| 1 |
4 (2.5) |
18 (30.0) |
32 (40.0) |
| 2 |
3 (1.9) |
35 (58.3) |
46 (57.5) |
| Papilla bleeding index, No. (%) |
0 |
41 (25.6) |
8 (13.3) |
9 (11.3) |
| 1 |
78 (48.8) |
22 (36.7) |
13 (16.3) |
| 2 |
37 (23.1) |
23 (38.3) |
41 (33.7) |
| 3 |
4 (2.5) |
7 (11.7) |
17 (9.3) |
| Mobility, No. (%) |
No |
140 (87.5) |
35 (58.3) |
46 (57.5) |
| Yes |
20 (12.5) |
25 (41.7) |
34 (42.5) |
| Oral health, No. (%) |
0 |
5 (3.1) |
9 (15) |
10 (12.5) |
| 1 |
16 (10.0) |
16 (26.7) |
11 (13.8) |
| 2 |
79 (49.4) |
24 (40.0) |
40 (50.0) |
| 3 |
60 (37.5) |
11 (18.3) |
19 (23.8) |
The percentage of smokers in generalized periodontitis was 18%, while that of smokers in localized periodontitis and gingivitis was 10% and 7.5%, respectively.
The results of using the continuation-ratio logistic model revealed that variables such as Al, PI, PD, ABL, and oral health have a significant effect on the occurrence of higher levels of periodontitis (localized and generalized) compared to gingivitis (Table 2). These findings are consistent with the results of identifying important variables based on the ordinal forest model. In the ordinal forest model, ABL, AL, PD, oral health, and PI were identified as the most important variables, respectively (Table 3 and Figure 1).
Table 2.
Variable Coefficients Based on the Continuation-ratio Logistic Model
|
Variable
|
Coefficients
|
Standard Error
|
P
Value
|
| (Intercept)1 |
8.72 |
4.73 |
0.065 |
| (Intercept)2 |
19.49 |
6.16 |
< 0.001 |
| Age |
0.03 |
0.04 |
0.471 |
| Attachment loss |
-7.05 |
1.85 |
< 0.001 |
| Plaque index |
-0.11 |
0.04 |
0.007 |
| Probing pocket depth |
-2.22 |
0.83 |
0.007 |
| Gender |
|
|
|
| Female (Reference) |
|
|
|
| Male |
0.96 |
1.15 |
0.400 |
| Smoking |
|
|
|
| Yes |
|
|
|
| No |
1.82 |
1.59 |
0.252 |
| Gingival index |
|
|
|
| Grade I (Reference) |
|
|
|
| Grade II |
1.99 |
1.42 |
0.162 |
| Grade III |
2.19 |
1.54 |
0.154 |
| Grade IV |
3.47 |
2.04 |
0.089 |
| Alveolar bone loss |
|
|
|
| 0 (Reference) |
|
|
|
| 1 |
-8.12 |
2.17 |
< 0.001 |
| 2 |
3.81- |
2.00 |
0.057 |
| Mobility |
|
|
|
| Yes (Reference) |
|
|
|
| No |
1.79 |
1.14 |
0.116 |
| Papilla bleeding index |
| 0 (Reference) |
|
|
|
| 1 |
2.63 |
1.37 |
0.054 |
| 2 |
0.59 |
1.41 |
0.674 |
| 3 |
1.96 |
2.20 |
0.371 |
| Oral health |
|
|
|
| 0 (Reference) |
|
|
|
| 1 |
7.64 |
2.40 |
0.001 |
| 2 |
6.43 |
1.97 |
0.001 |
| 3 |
19.41 |
4.46 |
< 0.001 |
Note. Reference category for ordinal response: Gingivitis.
Table 3.
Coefficients of Important Variables Based on the Ordinal Forest Model
|
|
Variable Important
|
| Alveolar bone loss |
0.2449 |
| Attachment loss |
0.0606 |
| Probing pocket depth |
0.0586 |
| Oral health |
0.0496 |
| Plaque index |
0.0396 |
| Papilla bleeding index |
0.0085 |
| Gingival index |
0.0048 |
| Mobility |
0.0042 |
| Gender |
0.0004 |
| Smoking |
0.0002 |
| Age |
0.0001 |
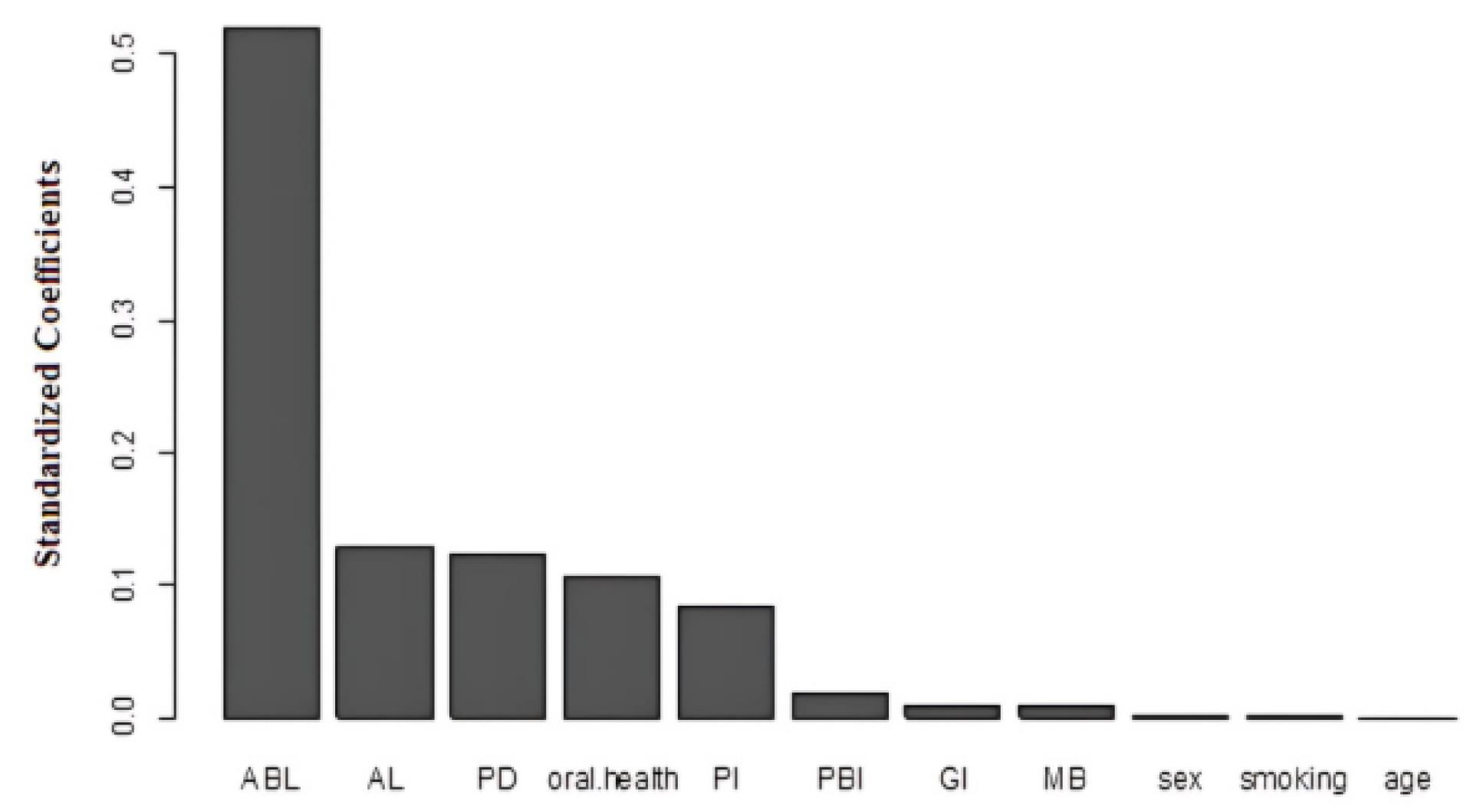
Figure 1.
Bar Plot of Important Variables for the Ordinal Forest Model
.
Bar Plot of Important Variables for the Ordinal Forest Model
A coefficient of 0.96 was obtained for men (women in the reference category) in the continuation model. This coefficient shows that male patients are more likely to have higher levels of periodontitis (localized and generalized than gingivitis) compared to females with exp coefficient (0.96). Therefore, male patients are about 2.5 times more likely to be classified as having high-stage periodontitis.
The continuation ratio model gives a coefficient of 19.41 for oral health (0 as reference category: excellent, 3 as poor oral hygiene). This coefficient implies that patients with poor oral hygiene status are more likely to have higher levels of periodontitis (localized and generalized rather than gingivitis) compared to patients with higher levels of oral hygiene by a factor of exp (19.41). Thus, patients with poor oral hygiene status are more likely to be classified in the high stage of periodontitis.
The results of comparing the predictive performance of continuation-ratio logistic and ordinal forest models are presented in Table 4, confirming the higher predictive ability of the ordinal forest model compared to the continuation-ratio logistic model in all indices. The ordinal forest model could correctly predict the different stages of the disease in periodontitis patients with 87% accuracy.
Table 4.
Kappa, Gamma, Somers’d, and Accuracy Indices for Model Comparison
|
|
Gamma |
Somers’d |
Kappa |
Accuracy |
| Ordinal forest model |
0.99 |
0.89 |
0.79 |
0.87 |
| Continuation-ratio logistic model |
0.95 |
0.83 |
0.67 |
0.80 |
Discussion
Accurate diagnosis of periodontitis plays an important role in determining treatment strategies that have a significant impact on the health of patients. Despite advances in treatment, no significant progress has been made in the diagnosis and prognosis of the disease, and diagnosis is usually highly dependent on empirical evidence. Therefore, this study sought to predict different levels of periodontitis and to identify the most important variables influencing it using logistic and ordinal forest models with a continuation ratio. The results of comparing the methods using the same test and training sets showed that the ordinal forest model had a better performance (accuracy of 0.87%) for classifying periodontal disease.
Various studies have been performed to classify periodontitis using different approaches, most of which do not take into account the ordinal nature of the response in the prediction and scoring indicators. For example, Youssif et al (27) applied an artificial neural network model to classify periodontitis at three levels using 11 input variables. The accuracy of the model in correctly identifying different periodontal diseases based on 30 samples was reported to be 100%.
Ozden et al (21) developed prediction models (output variables at 6 levels using 10 input variables) using an SVM, DT, and artificial neural networks. The results of implementing these methods on 150 patients, including 100 patients in the training set and 50 patients in the test set, revealed that the DT and SVM models generally had better performance with an overall accuracy of 98%.
Farhadian et al (17) conducted a study to develop a decision support system based on SVMs for the diagnosis of various periodontal diseases. The data included information on 300 patients, output variables at three nominal levels, and 11 input variables. The SVM model based on the RBF kernel had the best performance in the test set with an overall accuracy of 88.7%.
According to the results of the ordinal forest model, ABL was identified as the most important variable in the classification of periodontitis. Evidence confirmed that the extent of ABL can indicate the potential for tooth loss and the need for more aggressive treatment measures (28).
Probing PD plays a critical role in the classification and diagnosis of periodontal disease, according to the results of this study. The classification systems emphasize that probing depth is a critical indicator of periodontal health, with deeper pockets indicating more severe disease (29).
As poor oral hygiene is a major factor in the development and progression of periodontal disease, the results of the study confirm that oral health plays a significant role in the severity of periodontal disease. Therefore, maintaining good oral health is crucial in preventing and managing the severity of periodontal disease. Effective oral hygiene practices, regular dental visits, and awareness of the impact of the disease on overall health can significantly reduce the risks associated with periodontal disease (30).
Male gender is also associated with more severe periodontal disease than female gender. The preponderance of evidence suggests that male gender is an independent risk factor for more severe periodontal disease, probably due to a combination of poorer oral hygiene behaviors, differences in immune function, and other as yet unknown biological factors (31).
Although smoking was not identified as a significant variable, the results demonstrated that smoking is associated with increased severity of periodontitis. The association between smoking and disease severity may be due to the effect of smoking on the immune response and the promotion of the growth of pathogenic bacteria in the oral cavity (32).
Note, however, that the same test set was used to evaluate model predictive performance. In this regard, it is recommended that further studies evaluate the performance of the prediction model on the external dataset and determine its usability in clinical practice. In addition, because the prevalence of PD in clinical patient groups may differ from that in the national population, the distribution of data may be biased or unbalanced. These issues increase the risk of modelling bias and reduce model reliability, limiting the general use of predictive models.
Using an accurate model for predicting periodontitis, in addition to being highly predictive, maintains the ordinal nature of the response and can assist inexperienced dentists. In fact, using such systems can reduce fear (due to the lack of knowledge and skills, or loneliness) and increase self-confidence, especially in young doctors. These systems can be developed by designing and using decision-making systems in portable physician assistant devices or computers, and their development and evolution can lead to the satisfaction of the stakeholders of healthcare systems. Medical departments and offices provided real-time medical tools based on these prediction models to clinicians to make more reliable diagnoses.
Conclusion
Due to the ordinal nature of different levels of the periodontics disease, it is suggested that models be used that can take into account the ordinal nature of the response in predicting and evaluating the effect of important variables. Ordinal RF is a well-suited machine learning technique for developing accurate predictive models of periodontal disease risk.
Author’s Contribution
Conceptualization: Maryam Farhadian, Parviz Torkzaban.
Data curation: Parisa Shokouhi.
Formal Analysis: Zahra Torkashvand, Maryam Farhadian.
Methodology: Maryam Farhadian.
Project administration: Maryam Farhadian.
Software: Zahra Torkashvand, Maryam Farhadian.
Supervision: Maryam Farhadian.
Writing – original draft: Maryam Farhadian, Parviz Torkzaban.
Writing – review & editing: Maryam Farhadian, Parviz Torkzaban, Parisa Shokouhi, Zahra Torkashvand.
Competing Interests
There is no conflict of interests.
Ethical Approval
This study was approved by the Research Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1398.154).
Funding
This study was supported by the Vice-Chancellor of Research and Technology of Hamadan University of Medical Sciences (Contractor No. 9802311768). Moreover, this study was approved by the Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1398.154).
References
- Highfield J. Diagnosis and classification of periodontal disease. Aust Dent J 2009; 54 Suppl 1:S11-26. doi: 10.1111/j.1834-7819.2009.01140.x [Crossref] [ Google Scholar]
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999; 4(1):1-6. doi: 10.1902/annals.1999.4.1.1 [Crossref] [ Google Scholar]
- Kaufman E. The new classification system of periodontal diseases and conditions. Dent Today 2001; 20(9):102-5. [ Google Scholar]
- Ahmed Zaki A. The new classification of periodontal diseases. BDJ Team 2020; 7(9):32-3. doi: 10.1038/s41407-020-0435-5 [Crossref] [ Google Scholar]
- Nazir M, Al-Ansari A, Al-Khalifa K, Alhareky M, Gaffar B, Almas K. Global prevalence of periodontal disease and lack of its surveillance. ScientificWorldJournal 2020; 2020:2146160. doi: 10.1155/2020/2146160 [Crossref] [ Google Scholar]
- Shi M, Wei Y, Hu W, Nie Y, Wu X, Lu R. The subgingival microbiome of periodontal pockets with different probing depths in chronic and aggressive periodontitis: a pilot study. Front Cell Infect Microbiol 2018; 8:124. doi: 10.3389/fcimb.2018.00124 [Crossref] [ Google Scholar]
- Reddy MS, Geurs NC, Jeffcoat RL, Proskin H, Jeffcoat MK. Periodontal disease progression. J Periodontol 2000; 71(10):1583-90. doi: 10.1902/jop.2000.71.10.1583 [Crossref] [ Google Scholar]
- Ferreira MC, Dias‐Pereira AC, Branco‐de‐Almeida LS, Martins CC, Paiva SM. Impact of periodontal disease on quality of life: a systematic review. J Periodontal Res 2017; 52(4):651-65. doi: 10.1111/jre.12436 [Crossref] [ Google Scholar]
- Thomson WM, Sheiham A, Spencer AJ. Sociobehavioral aspects of periodontal disease. Periodontol 2000 2012; 60(1):54-63. doi: 10.1111/j.1600-0757.2011.00405.x [Crossref] [ Google Scholar]
- Ng SK, Leung WK. Oral health-related quality of life and periodontal status. Community Dent Oral Epidemiol 2006; 34(2):114-22. doi: 10.1111/j.1600-0528.2006.00267.x [Crossref] [ Google Scholar]
- Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control 2008; 19(9):895-907. doi: 10.1007/s10552-008-9163-4 [Crossref] [ Google Scholar]
- Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med 2008; 23(12):2079-86. doi: 10.1007/s11606-008-0787-6 [Crossref] [ Google Scholar]
- Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K. Periodontitis and diabetes: a two-way relationship. Diabetologia 2012; 55(1):21-31. doi: 10.1007/s00125-011-2342-y [Crossref] [ Google Scholar]
- Tse SY. Diabetes mellitus and periodontal disease: awareness and practice among doctors working in public general out-patient clinics in Kowloon West Cluster of Hong Kong. BMC Fam Pract 2018; 19(1):199. doi: 10.1186/s12875-018-0887-2 [Crossref] [ Google Scholar]
- Wiebe CB, Putnins EE. The periodontal disease classification system of the American Academy of Periodontology--an update. J Can Dent Assoc 2000; 66(11):594-7. [ Google Scholar]
- Kim EH, Kim S, Kim HJ, Jeong HO, Lee J, Jang J. Prediction of chronic periodontitis severity using machine learning models based on salivary bacterial copy number. Front Cell Infect Microbiol 2020; 10:571515. doi: 10.3389/fcimb.2020.571515 [Crossref] [ Google Scholar]
- Farhadian M, Shokouhi P, Torkzaban P. A decision support system based on support vector machine for diagnosis of periodontal disease. BMC Res Notes 2020; 13(1):337. doi: 10.1186/s13104-020-05180-5 [Crossref] [ Google Scholar]
- Chen CK. The classification of cancer stage microarray data. Comput Methods Programs Biomed 2012; 108(3):1070-7. doi: 10.1016/j.cmpb.2012.07.001 [Crossref] [ Google Scholar]
- Wolf BJ, Slate EH, Hill EG. Ordinal Logic Regression: a classifier for discovering combinations of binary markers for ordinal outcomes. Comput Stat Data Anal 2015; 82:152-63. doi: 10.1016/j.csda.2014.08.013 [Crossref] [ Google Scholar]
- Javali SB, Pandit PV. A comparison of ordinal regression models in an analysis of factors associated with periodontal disease. J Indian Soc Periodontol 2010; 14(3):155-9. doi: 10.4103/0972-124x.75909 [Crossref] [ Google Scholar]
- Ozden FO, Özgönenel O, Özden B, Aydogdu A. Diagnosis of periodontal diseases using different classification algorithms: a preliminary study. Niger J Clin Pract 2015; 18(3):416-21. doi: 10.4103/1119-3077.151785 [Crossref] [ Google Scholar]
- Janitza S, Tutz G, Boulesteix AL. Random forest for ordinal responses: prediction and variable selection. Comput Stat Data Anal 2016; 96:57-73. doi: 10.1016/j.csda.2015.10.005 [Crossref] [ Google Scholar]
- Singh V, Dwivedi SN, Deo SVS. Ordinal logistic regression model describing factors associated with extent of nodal involvement in oral cancer patients and its prospective validation. BMC Med Res Methodol 2020; 20(1):95. doi: 10.1186/s12874-020-00985-1 [Crossref] [ Google Scholar]
- Archer KJ, Williams AA. L1 penalized continuation ratio models for ordinal response prediction using high-dimensional datasets. Stat Med 2012; 31(14):1464-74. doi: 10.1002/sim.4484 [Crossref] [ Google Scholar]
- Hornung R. Ordinal forests. J Classif 2019; 11:2-14. doi: 10.1007/s00357-018-9302-x [Crossref] [ Google Scholar]
- Archer KJ, Hou J, Zhou Q, Ferber K, Layne JG, Gentry AE. ordinalgmifs: an R package for ordinal regression in high-dimensional data settings. Cancer Inform 2014; 13:187-95. doi: 10.4137/cin.s20806 [Crossref] [ Google Scholar]
- Youssif AA, Gawish AS, Moussa ME. Automated periodontal diseases classification system. Int J Adv Comput Sci Appl 2012; 3(1):40-8. doi: 10.14569/ijacsa.2012.030106 [Crossref] [ Google Scholar]
- Gul SS. Prevalence and severity of circumferential alveolar bone loss using CBCT images: a retrospective study of 20,620 surfaces of 5155 teeth. Diagnostics (Basel) 2024; 14(5):507. doi: 10.3390/diagnostics14050507 [Crossref] [ Google Scholar]
- Heitz-Mayfield LJ. Conventional diagnostic criteria for periodontal diseases (plaque-induced gingivitis and periodontitis). Periodontol 2000. 2024. 10.1111/prd.12579.
- Bhargava N, Jadhav A, Kumar P, Kapoor A, Mudrakola DP, Singh S. Oral health-related quality of life and severity of periodontal disease. J Pharm Bioallied Sci 2021; 13(Suppl 1):S387-90. doi: 10.4103/jpbs.JPBS_588_20 [Crossref] [ Google Scholar]
- Al-Abdaly MM, AlQahtani HS, Al-Qahtani SS. The impact of age and gender on severity and types of periodontal diseases among patients from two regions in Saudi Arabi. Open J Stomatol 2019; 9(3):39-50. doi: 10.4236/ojst.2019.93005 [Crossref] [ Google Scholar]
- Koshi E, Rajesh S, Koshi P, Arunima PR. Risk assessment for periodontal disease. J Indian Soc Periodontol 2012; 16(3):324-8. doi: 10.4103/0972-124x.100905 [Crossref] [ Google Scholar]