Avicenna J Dent Res. 15(4):178-182.
doi: 10.34172/ajdr.1721
Case Report
Intraosseous Mucoepidermoid Carcinoma of the Mandible Arising From a Cystic Lesion: A Case Report
Mohammad Reza Jamalpour 1  , Arash Dehghan 2, Reza Nadripour 1, Faryad Fatehi 1, Hossein Nahidfar 1, Hamid Naderi 1, *
, Arash Dehghan 2, Reza Nadripour 1, Faryad Fatehi 1, Hossein Nahidfar 1, Hamid Naderi 1, * 
Author information:
1Department of Oral & Maxillofacial Surgery, School of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Pathology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Mucoepidermoid carcinoma (MEC) can be found as a primary intraosseous lesion, accounting for 2-3% of head and neck MEC. The mandibular premolar-molar region is the most common site, up to 50% of which is associated with dental cysts and/or impacted teeth. Histopathologically, it is classified into low, intermediate, and high grades. The best modality of treatment for intraosseous MEC is radical surgical resection. Radiotherapy is recommended to improve the prognosis in patients with positive margins, positive node disease, and moderate- and high-grade lesions. This paper reports a rare case of intraosseous MEC of the mandible in a 17-year-old female, discovered after the panoramic radiography of the jaws and treated by the segmental resection of the mandible through transoral approach and adjuvant radiotherapy.
Keywords: Mucoepidermoid carcinoma, Intraosseous, Adjuvant radiotherapy, Impacted teeth
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Jamalpour MR, Dehghan A, Nadripour R, Fatehi F, Nahidfar H, Naderi H. Intraosseous mucoepidermoid carcinoma of the mandible arising from a cystic lesion: a case report. Avicenna J Dent Res. 2023; 15(4):178-182. doi:10.34172/ajdr.1721
Background
Mucoepidermoid carcinoma (MEC) is commonly associated with the salivary glands and accounts for 5-10% of all salivary gland tumors (1,2). MEC can be found as a primary intraosseous lesion, accounting for 2-3% of head and neck MEC, and occurs more frequently in the posterior region of the mandible (3). The mandibular premolar-molar region is the most common site, with up to 50% associated with dental cysts and/or impacted teeth (4). The association with cysts or impacted teeth can confirm the theory that the odontogenic epithelium leads to the creation of mucous secretory cells that undergo malignant transformation (5).
The present article aims to report a case of the intraosseous MEC of the mandible managed by the segmental resection of the mandible via the transoral approach, followed by adjuvant radiotherapy, and to review the literature to further understand the biological behavior, diagnosis, and management of these neoplasms.
Case Report
A 17-year-old female patient was referred by the dentist when a radiolucent lesion on the left posterior mandible was discovered after a panoramic X-ray of the jaws. At the extraoral physical examination, there were no signs of increased volume and/or facial asymmetry. At the transoral examination, the lesion presented with lingual cortical expansion, mucosa with normal coloration, and no dental displacement. The swelling was soft to firm. The patient was noted to have pain spontaneously and with the palpation of the left retromolar pad area. Aspiration puncture was negative. Panoramic radiographic examination revealed a radiolucent, unilocular, well-defined lesion extending from the distal part of tooth #37 to the ascending region of the ramus. Tooth #38 was evidently in the form of a transverse impaction in the lesion. In the cone-beam computed tomography (CBCT) view, the buccal and lingual expansion of the lesion, along with the destruction of the lingual wall, was evident. The roof of the inferior alveolar canal was destroyed in some areas. No root resorption and tooth displacement were observable (Figure 1).
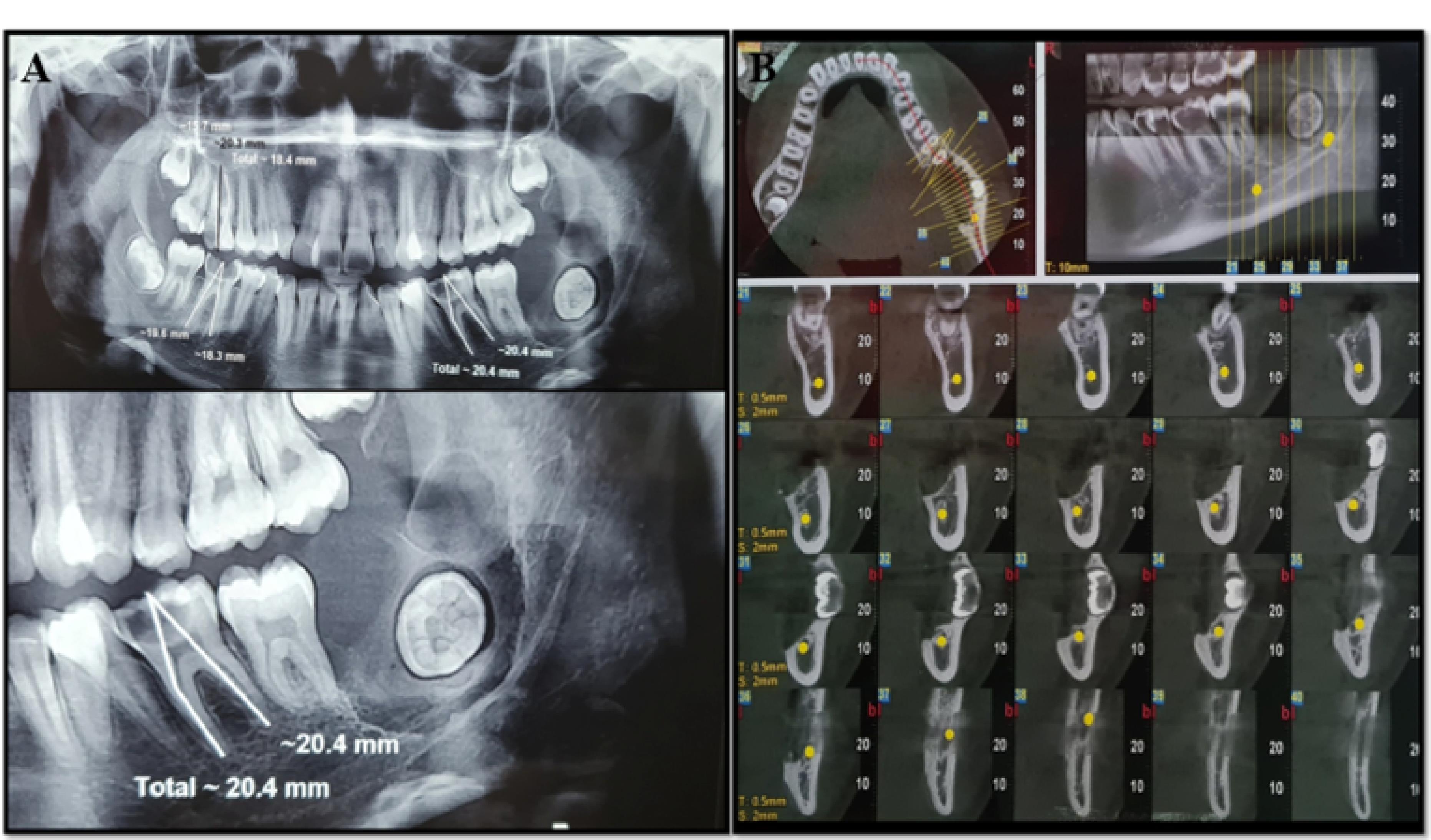
Figure 1.
Radiographic view, (A). Panoramic view. A radiolucent, unilocular, well-defined lesion extending from the distal part of tooth #37 to the ascending region of the ramus, (B). CBCT view. The buccal and lingual expansion of the lesion along with the destruction of the lingual wall is evident.
.
Radiographic view, (A). Panoramic view. A radiolucent, unilocular, well-defined lesion extending from the distal part of tooth #37 to the ascending region of the ramus, (B). CBCT view. The buccal and lingual expansion of the lesion along with the destruction of the lingual wall is evident.
An incisional biopsy was performed on the affected area, and low-grade intraosseous MEC, which was negative for lymphovascular invasion, tumoral tissue necrosis, and perineural invasion, was reported in histopathological evaluations, and an odontogenic cyst was identified as well. In the spiral computerized tomography (CT) scan with and without contrast of the face, neck, and lungs, a lytic lesion 12 × 8 mm in size was observed at the angle of the left mandible. The enhancement of the soft tissue, the medial part of the bone lesion in the involved area, 13 × 22 mm in size with relatively defined boundaries, was detected while not observing cervical lymphadenopathy and pulmonary involvement.
The clinical differential diagnosis encompasses odontogenic tumors related to an impacted tooth, including unicystic ameloblastoma, ameloblastic fibroma, keratocystic odontogenic tumor, and calcifying epithelial odontogenic tumor. In addition, a dentigerous cyst, which is the second most common odontogenic cyst, and glandular odontogenic cyst are considered differential diagnoses.
The surgical planning was en block resection with continuity defect (Rc − CD) with a safety margin through the transoral approach and vestibular incision from the ascending region of the left ramus to the midline of the mandible (Figure 2). Resection was performed from the distal area of tooth #35 to the condylar base, reconstruction of the area with a titanium macro plate, and fixation of the arch bar (Figure 3). Low-grade MEC was reported according to permanent pathology outcomes. The tumor size was 2.5 × 2 × 1 cm, which was negative for lymphovascular invasion, tumoral tissue necrosis, and perineural invasion. The distance of all surgical margins was < 5 mm. The mandible bone was free of neoplastic involvement (Figure 4). Subsequently, the patient was submitted to radiotherapy to mitigate the possibility of the lesion and recurrence. According to the spiral CT scan of the thorax, head, and neck with and without contrast in the 6-month and 1-year follow-up, there was no evidence of recurrent and metastatic lesions. The next treatment plan was to reconstruct the resected mandible with patient-specific prostheses.
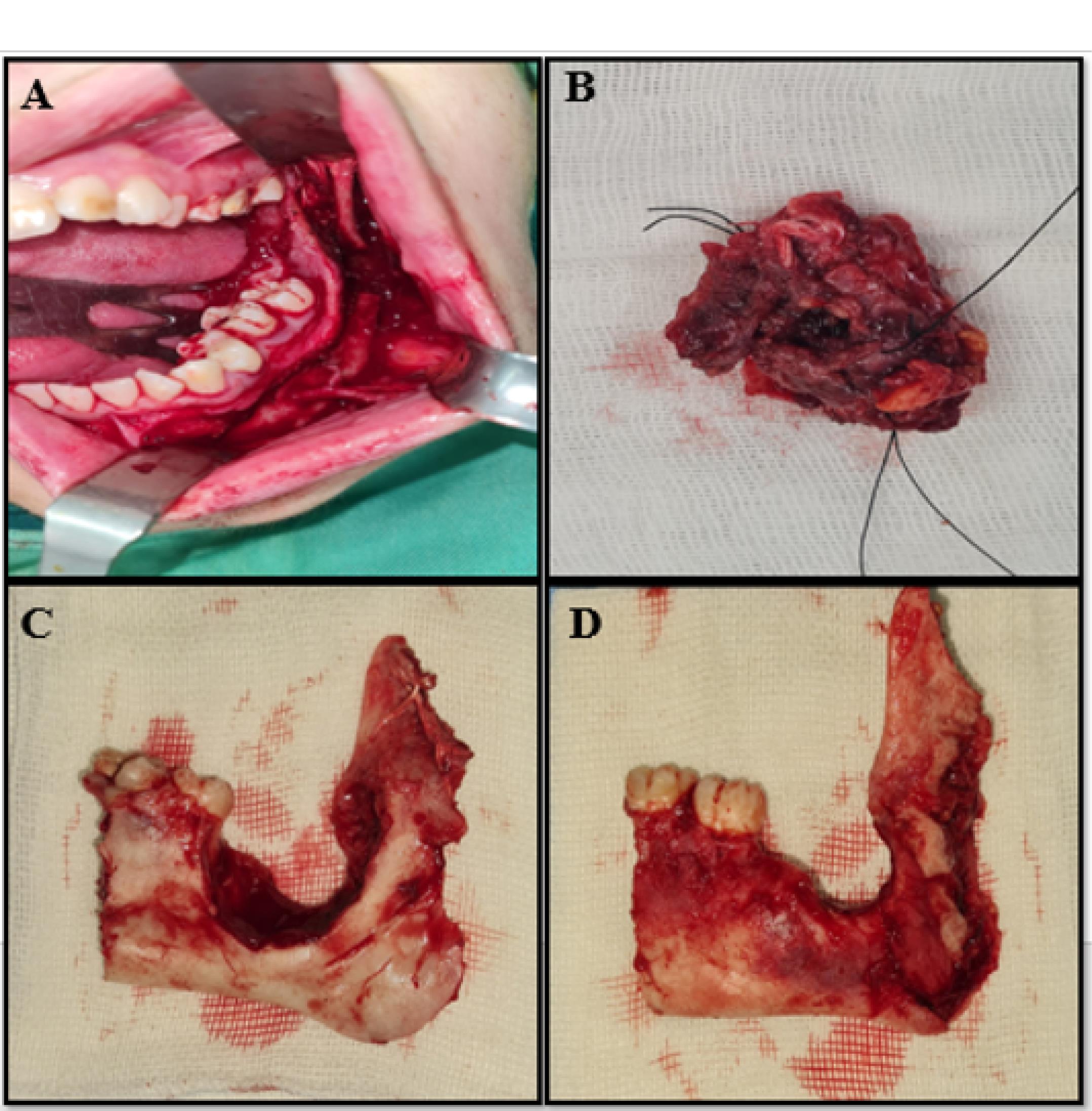
Figure 2.
Pathologic lesion view, (A) Transoral approach and vestibular incision, (B) Resected tumor, (C) Buccal view, (D) Lingual view.
.
Pathologic lesion view, (A) Transoral approach and vestibular incision, (B) Resected tumor, (C) Buccal view, (D) Lingual view.
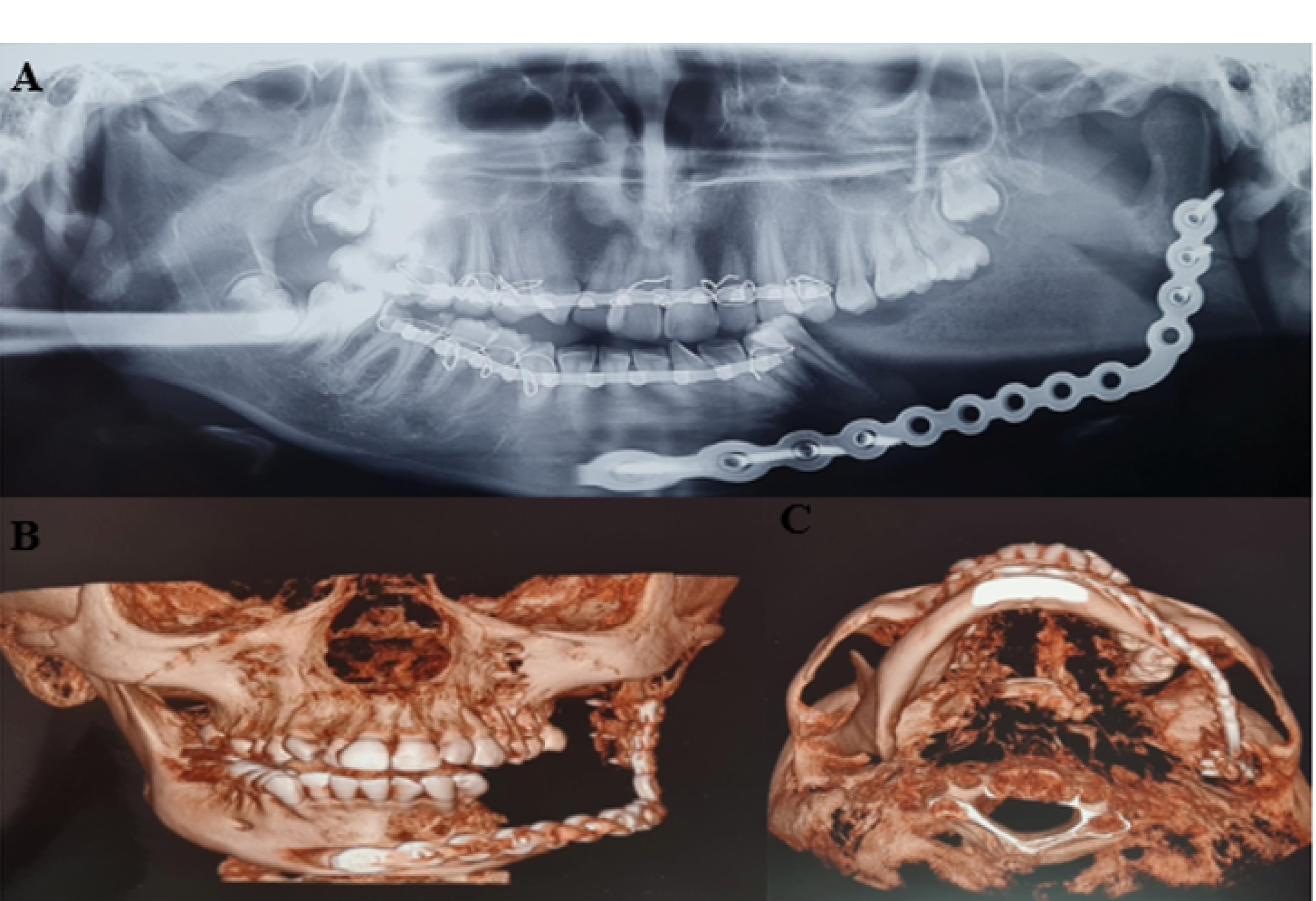
Figure 3.
Follow up after 6 months, (A) Panoramic view, (B) 3d reconstruction of CBCT, coronal view. C. 3d reconstruction of CBCT, axial view.
.
Follow up after 6 months, (A) Panoramic view, (B) 3d reconstruction of CBCT, coronal view. C. 3d reconstruction of CBCT, axial view.
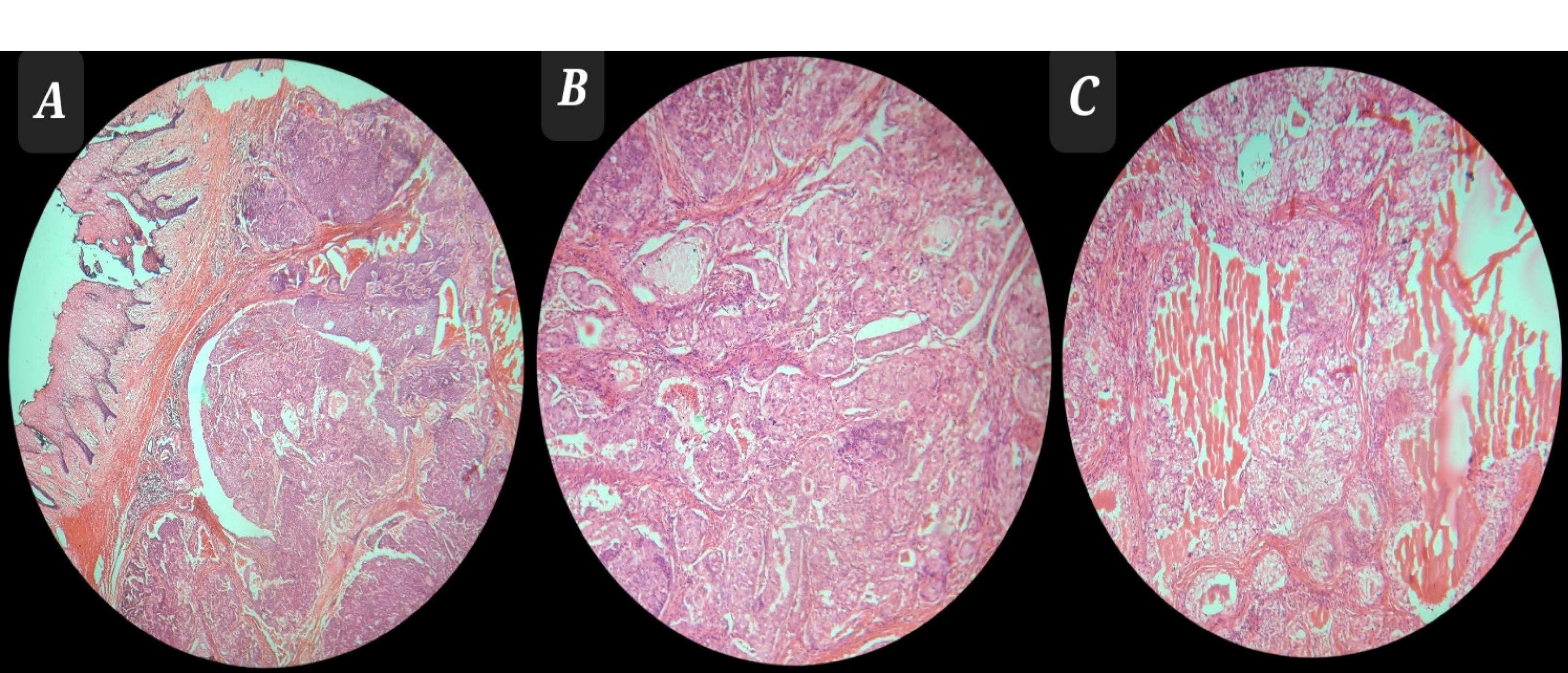
Figure 4.
(A) Neoplastic Tissue With an Infiltrative Growth Pattern Extended to Mucosa (H&E Stain × 0.4), (B) Mixture of Neoplastic Cells, Including Clear and Squamous Cells With an Infiltrative Growth Pattern (H&E Stain × 2.5), and (C) Mixture of Clear and Squamous Cells Arranged in Cellular Clusters (H&E Stain × 2.5) Note. H&E: Hematoxylin and eosin.
.
(A) Neoplastic Tissue With an Infiltrative Growth Pattern Extended to Mucosa (H&E Stain × 0.4), (B) Mixture of Neoplastic Cells, Including Clear and Squamous Cells With an Infiltrative Growth Pattern (H&E Stain × 2.5), and (C) Mixture of Clear and Squamous Cells Arranged in Cellular Clusters (H&E Stain × 2.5) Note. H&E: Hematoxylin and eosin.
Discussion
MEC usually occurs in the fourth to sixth decade of life with a greater tendency in women than in men (6). It involves the lower jaw twice as much as the upper jaw (2). As the tumor tends to grow during puberty, the hormonal influence of the salivary glands was suggested as an etiological factor (7). The painless swelling of the jaw is the most common manifestation, which sometimes manifests with pain, paresthesia, numbness, and loose teeth (8).
Although the exact pathogenesis of MEC is unknown, there are several current theories of its origin. The probable origins for these lesions are the ectopic salivary gland tissue (the remnants of embryonic salivary glands trapped within the bone), the transformation of mucous cells found in odontogenic cysts, and maxillary sinuses or submucosal and mucosal glands with intraosseous extension (9).
Histopathologically, it is classified into low (48%), intermediate (38.7%), and high (13.3%) grades. These three histopathological grades are based on the degree of cytological atypia, the amount of cyst formation, and the relative number of mucous, epidermoid, and intermediate cells (10). Low-grade tumors have a higher ratio of mucous cells and are less aggressive lesions, while high-grade tumors have a smaller proportion of mucous cells and are considered to be more malignant tumors with poorer prognoses (11). Brookstone and Huvos (8), based on radiology, proposed a staging system based on the condition of the overlying bone (Table 1).
Table 1.
Clinical Staging of Central Salivary Gland Tumor Including Central Mucoepidermoid Carcinoma
|
Stage
|
Condition of Overlying Bone
|
| Stage I |
Without bony expansion and rupture of cortical plate |
| Stage II |
Intact cortical bone that has undergone some degree of expansion |
| Stage III |
Rupture of cortical plate or nodal involvement |
Although rare, intraosseous carcinoma appearing in the bones of the jaw, which was first described by Loos in 1913 as a central epidermoid carcinoma, is a recognized clinical entity (12). Later, Waldron and Mustoe (13) suggested that intraosseous MEC is included in the classification of “primary intraosseous carcinoma” (PIOC) as type 4 (Table 2). This is based on the fact that the MEC of the jaw arises from the epithelial remnants of an odontogenic cyst and is histologically similar to salivary MEC.
Table 2.
Waldron and Mustoe modification of the WHO Classification of Primary Intraosseous Carcinoma
|
Type
|
Modified Classification of PIOC (WHO, 2005)
|
| Type 1 |
PIOC ex odontogenic cyst |
| Type 2a |
Malignant ameloblastoma |
| Type 2b |
Ameloblastic carcinoma arising de novo, ex-ameloblastoma, or
ex-odontogenic cyst |
| Type 3 |
PIOC arising de novo |
| Type 3a |
Keratinizing type |
| Type 3b |
Nonkeratinizing type |
| Type 4 |
Intraosseous mucoepidermoid carcinoma |
Note. WHO: World Health Organization; PIOC: Primary intraosseous carcinoma.
Our patient had an impacted wisdom tooth surrounded by the lesion, which may indicate the possibility of the neoplastic transformation of the cyst wall into a malignant nonodontogenic tumor. The patient had expanded perforated cortices with radiographic evidence of bone destruction classified as PIOC type 4. Pathologic evaluation exhibits prominent cystic formation, minimal cellular atypia, and a relatively high proportion of mucosal cells, and these criteria represent a low-grade tumor. None was found in a thorough search for the primary tumor elsewhere by careful clinical and other diagnostic methods. According to the staging system based on the condition of the overlying bone, this lesion belongs to stage III.
The best modality of treatment for intraosseous MEC is surgery. In a review of 64 patients, Brookstone and Huvos observed 40% relapses after conservative surgical modalities. In the group treated by segmental resection with or without adjuvant treatment, recurrence occurred in only 4% of cases. Adjuvant therapy such as radiotherapy and/or chemotherapy is recommended for high-grade tumors (8,14). Chemotherapy in the treatment of MEC is reserved for cases of invasive or metastatic lesions that are not amenable to surgery or radiation therapy (15).
In this case, we performed transoral surgical resection. The main advantage of this method, especially in a young person, is maintaining the esthetic and function of the lower lip, while in other published articles, the extraoral approach was used for tumor resection in similar extensive lesions. In 2019, Abt et al (16) performed surgery for the treatment of the intraosseous MEC of the mandible through a transoral approach, although the size of the lesion was smaller and its spread was less than in our case. Improvement in the survival rate has been reported in 5% of patients who underwent radiotherapy after surgery. Therefore, radiotherapy should be recommended to improve prognosis in patients with positive margins, positive node disease, and moderate- and high-grade lesions (17). In our patient, according to the staging system, the lesion is placed in stage III. In addition, according to pathology outcomes, the margins of the tumoral lesion were reported as close margins; thus, the patient underwent radiotherapy to reduce the possibility of tumor recurrence after surgery. Several studies (5,17-22) used the segmental resection of the mandible and adjuvant radiotherapy to treat a low-grade MEC of the mandible. Recurrence was reported in the case studied by Zhou et al (20).
Conclusion
Considering that most intraosseous MEC lesions are low-grade and less invasive, the clinical importance of these malignant tumors should never be underestimated. Surgical resection treatment, along with adjuvant treatment and detailed histopathological evaluations of the entire removed tissue, is highly helpful in the identification, treatment, and follow-up of these lesions.
Authors’ Contribution
Conceptualization: Mohammad Reza Jamalpour, Arash Dehghan.
Data curation: Hamid Naderi, Reza Nadripour.
Investigation: Faryad Fatehi, Hossein Nahidfar.
Methodology: Mohammad Reza Jamalpour, Arash Dehghan, Reza Nadripour.
Project administration: Mohammad Reza Jamalpour, Hamid Naderi.
Resources: Mohammad Reza Jamalpour.
Supervision: Mohammad Reza Jamalpour.
Validation: Mohammad Reza Jamalpour, Arash Dehghan.
Visualization: Mohammad Reza Jamalpour, Hamid Naderi.
Writing–original draft: Hamid Naderi.
Writing–review & editing: Hamid Naderi, Reza Nadripour.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
None to be declared.
Informed Consent
Verbal and written consent was obtained from the patients.
References
- Eversole LR. Mucoepidermoid carcinoma: review of 815 reported cases. J Oral Surg 1970; 28(7):490-4. [ Google Scholar]
- Gingell JC, Beckerman T, Levy BA, Snider LA. Central mucoepidermoid carcinoma Review of the literature and report of a case associated with an apical periodontal cyst. Oral Surg Oral Med Oral Pathol 1984; 57(4):436-40. doi: 10.1016/0030-4220(84)90165-8 [Crossref] [ Google Scholar]
- Pires FR, Paes de Almeida O, Lopes MA, Elias da Cruz Perez D, Kowalski LP. Central mucoepidermoid carcinoma of the mandible: report of four cases with long-term follow-up. Int J Oral Maxillofac Surg 2003; 32(4):378-82. doi: 10.1054/ijom.2002.0374 [Crossref] [ Google Scholar]
- Eversole LR, Sabes WR, Rovin S. Aggressive growth and neoplastic potential of odontogenic cysts: with special reference to central epidermoid and mucoepidermoid carcinomas. Cancer 1975; 35(1):270-82. doi: 10.1002/1097-0142(197501)35:1<270::aid-cncr2820350134>3.0.co;2-y [Crossref] [ Google Scholar]
- Bell D, Lewis C, El-Naggar AK, Weber RS. Primary intraosseous mucoepidermoid carcinoma of the jaw: reappraisal of the MD Anderson Cancer Center experience. Head Neck 2016; 38 Suppl 1:E1312-7. doi: 10.1002/hed.24219 [Crossref] [ Google Scholar]
- Sidoni A, D’Errico P, Simoncelli C, Bucciarelli E. Central mucoepidermoid carcinoma of the mandible: report of a case treated 13 years after first radiographic demonstration. J Oral Maxillofac Surg 1996; 54(10):1242-5. doi: 10.1016/s0278-2391(96)90361-4 [Crossref] [ Google Scholar]
- Caccamese JF Jr, Ord RA. Paediatric mucoepidermoid carcinoma of the palate. Int J Oral Maxillofac Surg 2002; 31(2):136-9. doi: 10.1054/ijom.2001.0221 [Crossref] [ Google Scholar]
- Brookstone MS, Huvos AG. Central salivary gland tumors of the maxilla and mandible: a clinicopathologic study of 11 cases with an analysis of the literature. J Oral Maxillofac Surg 1992; 50(3):229-36. doi: 10.1016/0278-2391(92)90317-s [Crossref] [ Google Scholar]
- Johnson B, Velez I. Central mucoepidermoid carcinoma with an atypical radiographic appearance. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106(4):e51-3. doi: 10.1016/j.tripleo.2008.04.029 [Crossref] [ Google Scholar]
- Rajendran R. Shafer’s Textbook of Oral Pathology. India: Elsevier; 2009.
- Kumar AN, Nair PP, Thomas S, Raman PS, Bhambal A. Mucoepidermoid carcinoma of sublingual gland: a malignant neoplasm in an uncommon region. BMJ Case Rep 2011;2011. 10.1136/bcr.02.2011.3864.
- Loos D. Central epidermoid carcinoma of the jaws. Dtsch Monatschr Zahnheilk 1913; 31:308. [ Google Scholar]
- Waldron CA, Mustoe TA. Primary intraosseous carcinoma of the mandible with probable origin in an odontogenic cyst. Oral Surg Oral Med Oral Pathol 1989; 67(6):716-24. doi: 10.1016/0030-4220(89)90014-5 [Crossref] [ Google Scholar]
- Nance MA, Seethala RR, Wang Y, Chiosea SI, Myers EN, Johnson JT. Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer 2008; 113(8):2082-9. doi: 10.1002/cncr.23825 [Crossref] [ Google Scholar]
- Ali SA, Memon AS, Shaik NA, Soomro AG. Mucoepidermoid carcinoma of parotid presenting as unilocular cyst. J Ayub Med Coll Abbottabad 2008; 20(2):141-2. [ Google Scholar]
- Abt NB, Lawler ME, Zacharias J, Lahey ET. Primary intraosseous mucoepidermoid carcinoma of the mandible: radiographic evolution and clinicopathological features. BMJ Case Rep 2019; 12(4):e224612. doi: 10.1136/bcr-2018-224612 [Crossref] [ Google Scholar]
- He Y, Wang J, Fu HH, Zhang ZY, Zhuang QW. Intraosseous mucoepidermoid carcinoma of jaws: report of 24 cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114(4):424-9. doi: 10.1016/j.oooo.2011.12.021 [Crossref] [ Google Scholar]
- Singh M, Nangia S, Cudahy T, Mir R. Adjuvant conundrum in central mucoepidermoid carcinoma of the mandible: case presentation and literature review. BMJ Case Rep 2018; 2018:bcr2018226380. doi: 10.1136/bcr-2018-226380 [Crossref] [ Google Scholar]
- de Freitas GB, de França AJB, Dos Santos ST, de Lima Júnior MO, da Fonte Neto AS, Bernardon P. Intraosseous mucoepidermoid carcinoma in the mandible. Case Rep Dent 2018; 2018:9348540. doi: 10.1155/2018/9348540 [Crossref] [ Google Scholar]
- Zhou CX, Chen XM, Li TJ. Central mucoepidermoid carcinoma: a clinicopathologic and immunohistochemical study of 39 Chinese patients. Am J Surg Pathol 2012; 36(1):18-26. doi: 10.1097/PAS.0b013e31822be0df [Crossref] [ Google Scholar]
- Merna C, Kita A, Wester J, Diaz-Aguilar D, Goldstein JD, Palma Diaz F. Intraosseous mucoepidermoid carcinoma: outcome review. Laryngoscope 2018; 128(5):1083-92. doi: 10.1002/lary.26832 [Crossref] [ Google Scholar]
- Martínez-Madrigal F, Pineda-Daboin K, Casiraghi O, Luna MA. Salivary gland tumors of the mandible. Ann Diagn Pathol 2000; 4(6):347-53. doi: 10.1053/adpa.2000.19395 [Crossref] [ Google Scholar]