Avicenna J Dent Res. 15(4):183-188.
doi: 10.34172/ajdr.1675
Case Report
Major Manifestations of Gorlin Syndrome in a Patient With Hemophilia A
Elham Hadadian Pour 1  , Amirhosein Haghir 2, Sirous Risbaf Fakour 3, *
, Amirhosein Haghir 2, Sirous Risbaf Fakour 3, * 
Author information:
1Department of Oral & Maxillofacial Surgery, Zahedan University of Medical Sciences, Zahedan, Iran
2Department of Neurosurgery, Mashhad University of Medical Sciences, Mashhad, Iran
3Oral and Dental Disease Research Center, Department of Oral and Maxillofacial Surgery, Zahedan University of Medical sciences, Zahedan, Iran
Abstract
Nevoid basal cell carcinoma syndrome (NBCCS) is a rare autosomal dominantly inherited condition in which constitutive pathway activity and tumor cell proliferation are disturbed due to defects in hedgehog signaling. This study reports an adult case of hemophilia A suspected of having Gorlin syndrome. There is no similar evidence in the literature to describe the association between these disorders. NBCCS is characterized by the development of multiple basal cell carcinomas (BCCs). The case presented in this study had two major manifestations of NBCCS, including the calcification of the falx cerebri and the presence of keratocystic odontogenic tumors (KOTs); however, no minor manifestations of NBCCS were found in this case. On the other hand, given the financial restrictions, it was impossible to perform a genetic examination to confirm the presence of NBCCS.
Keywords: Calcification, Falx cerebri, Gorlin syndrome, Odontogenic keratocyst
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Hadadian Pour E, Haghir A, Risbaf Fakour S. Major manifestations of Gorlin syndrome in a patient with hemophilia A. Avicenna J Dent Res. 2023; 15(4):183-188. doi:10.34172/ajdr.1675
Background
Nevoid basal cell carcinoma syndrome (NBCCS) is an infrequent autosomal dominantly inherited condition in which tumor cell proliferation and constitutive pathway activity are disturbed because of defects in hedgehog signaling (1). The prevalence of Gorlin syndrome has been estimated to be 1 per 57 000 to 164 000 persons without sex predilection, and it is dominant in lighter-pigmented persons (2).
Patients with established NBCCS are predisposed to developmental defects that lead to the formation of tumors, especially multiple basal cell carcinomas (BCCs) (3). Patched 1 gene (PTCH1), encoding the PTCH1 transmembrane receptor, plays an important role in the molecular pathogenesis of NBCCS. PTCH1 is also implicated in the sonic hedgehog signaling pathway (4). Calcification of the falx cerebri, the presence of BCCs, odontogenic keratocyst (OKC) or keratocystic odontogenic tumors (KOTs), and skeletal anomalies are the main manifestations of the condition (5,6). Cutaneous and central nervous system symptoms, developmental abnormalities, and skeletal malfunctions have been introduced as the lesser-known manifestations of the condition (2). Surgery and the use of the sonic hedgehog signaling inhibitor vismodegib have been reported as the main therapeutic options. The present study reports an adult case of hemophilia A suspected of having Gorlin syndrome.
Case Report
A 24-year-old male with severe hemophilia A referred to Khatam Al-Anbiya hospital, Zahedan, Iran. The patient had been diagnosed with severe hemophilia when he was three years old. He also had a history of brain trauma and had undergone a craniotomy at three years of age. Three years ago, the patient had been referred to dentistry due to intraoral inflammation (Figure 1) and had undergone an orthopantomography (OPG) and a computerized tomography (CT) scan.
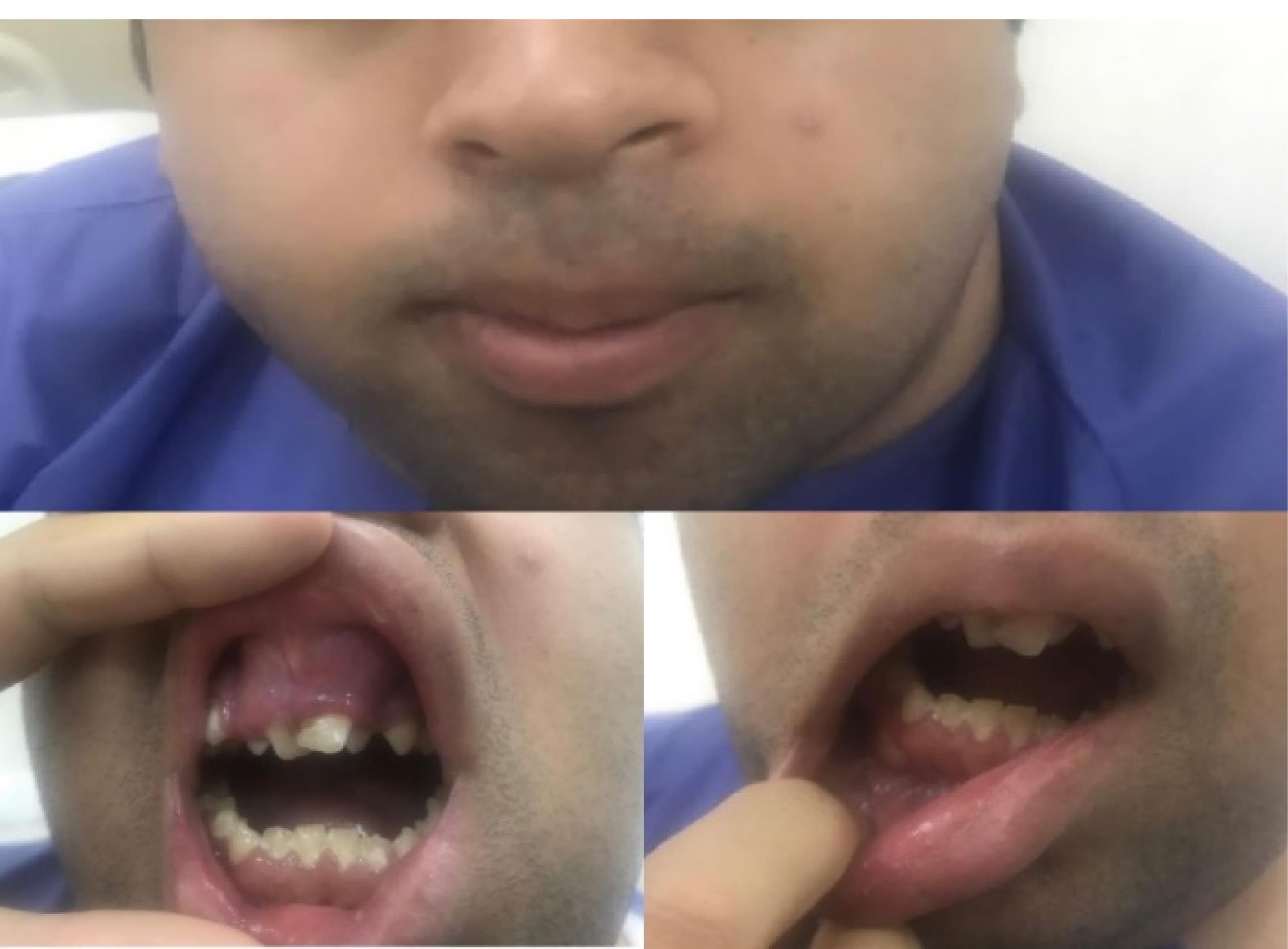
Figure 1.
Patient With Severe Hemophilia A and Intraoral Inflammation
.
Patient With Severe Hemophilia A and Intraoral Inflammation
Cranial radiography confirmed the previous craniotomy and showed the intraoral cysts (Figure 2). Figure 3 displays the preoperative OPG radiograph, depicting multiple cysts before and three years after cyst resection and bone regeneration.
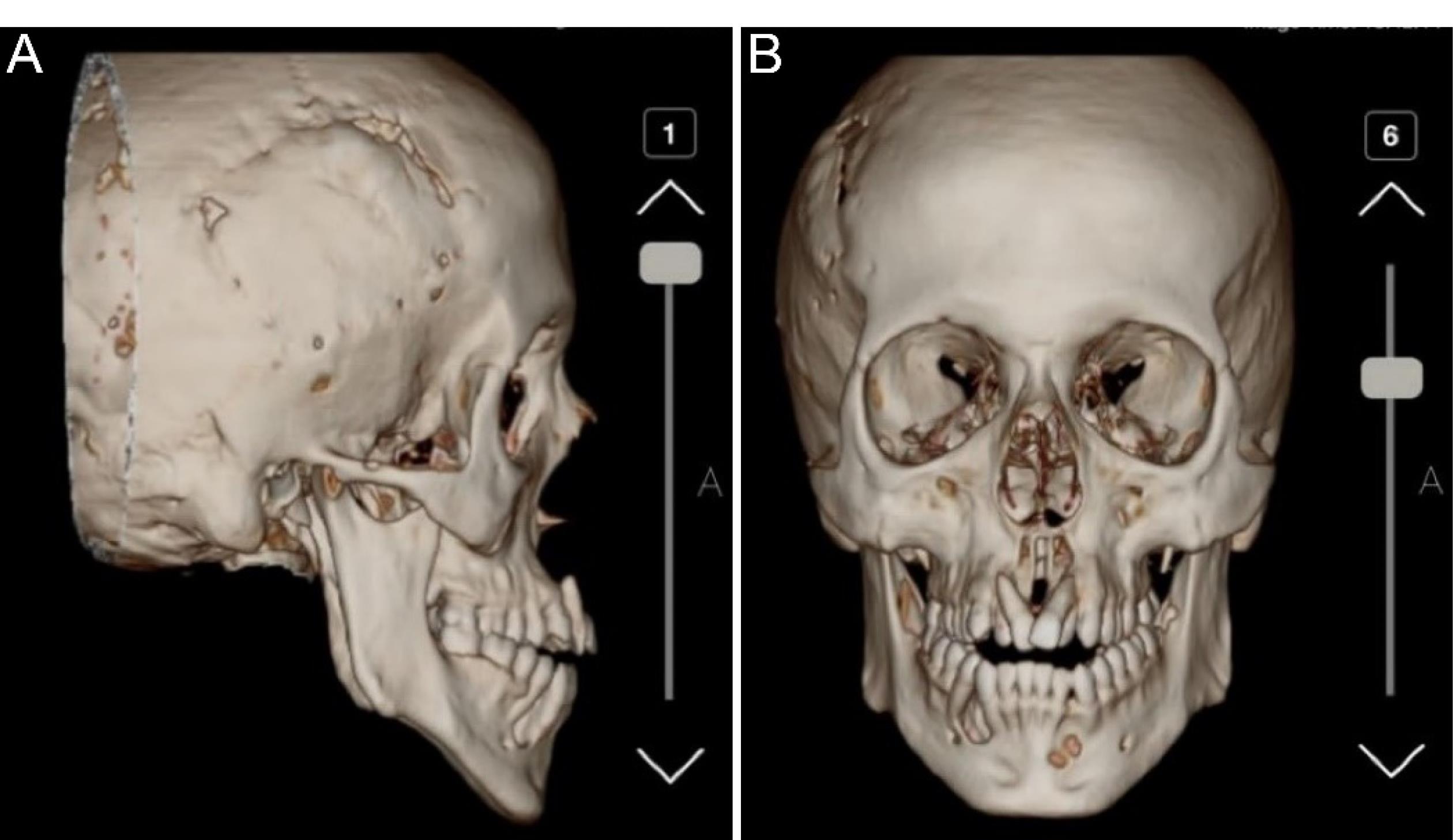
Figure 2.
Previous Craniotomy (A) and Intraoral Cysts (B) Detected by Computerized Tomography Scan
.
Previous Craniotomy (A) and Intraoral Cysts (B) Detected by Computerized Tomography Scan
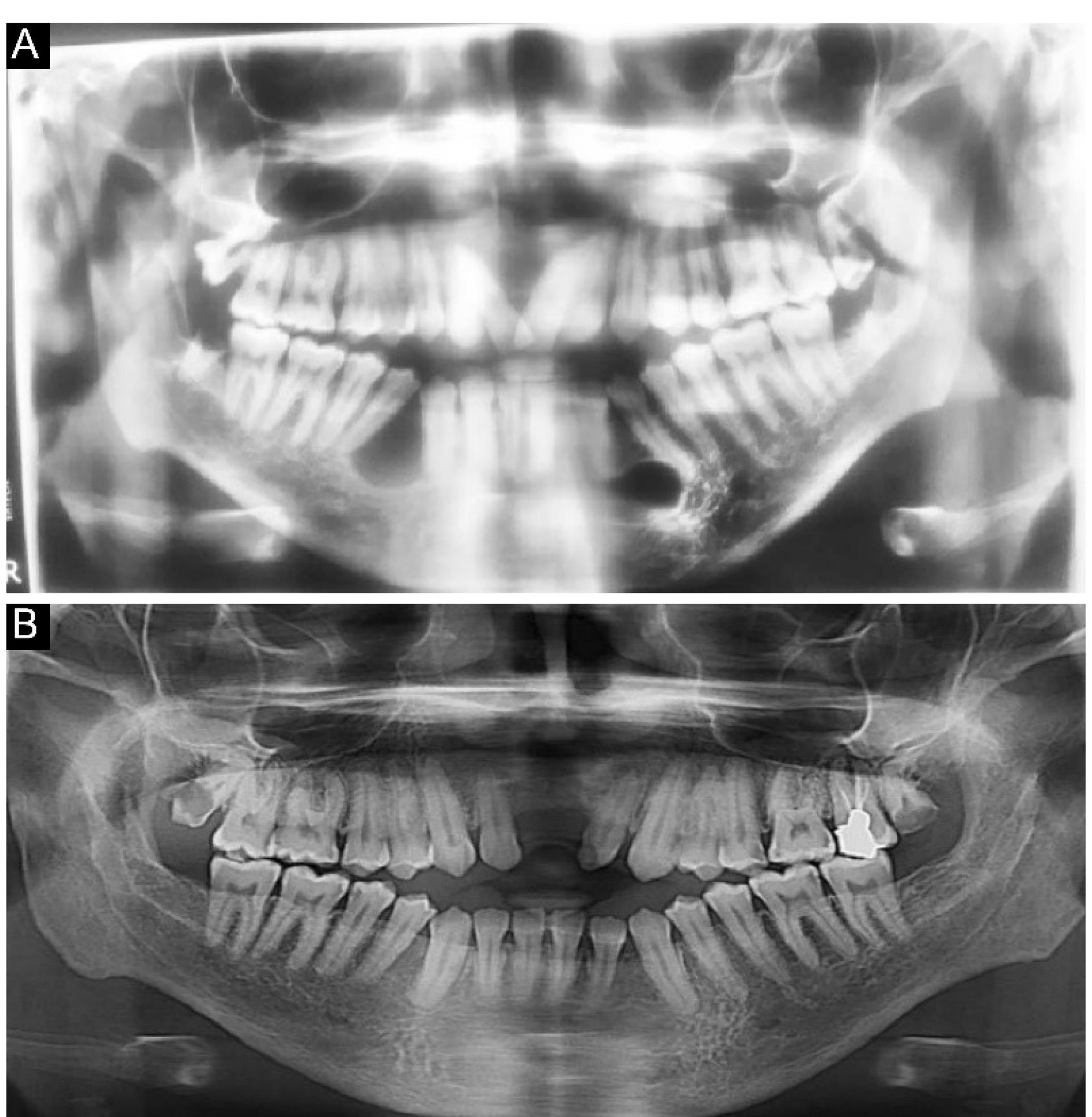
Figure 3.
Orthopantomogram Radiographs of Various Cysts Before (A) and Three Years After (B) the Surgery
.
Orthopantomogram Radiographs of Various Cysts Before (A) and Three Years After (B) the Surgery
Based on the OPG, there were unilocular radiolucent lesions with well-circumscribed corticated borders on the left side of the mandible in the area of the fourth and fifth teeth without evidence of expansion or destruction. Loss of lamina dura was observed at the periapical region of the second and third teeth, and partial root resorption was detected in the second and third molar teeth. There was a lesion on the left side of the mandible and a lesion on the right side of the mandible (both at the second and third sites), as well as a lesion in the maxilla (between the 2 right and 2 left teeth). Moreover, unilocular radiolucent lesions with well-circumscribed corticated borders were observed in front of the mandibular right ramus without evidence of expansion/destruction or impacts on the alveolar canal. On the right side of the mandible, unilocular radiolucent lesions with well-defined non-corticated borders were found in the area of the fourth and fifth teeth, which had caused the displacement and loss of lamina without evidence of root resorption. The differential diagnosis was myxoma, central giant cell granuloma (CGCG), and KOTs on the right and left sides of the mandible and ramus. Moreover, a well-circumscribed, non-corticated lesion was detected in front of the maxilla, which extended from the second tooth from one side to the second tooth on another side in the horizontal dimension and from the alveolar crest to the nasal floor in the vertical dimension. The differential diagnoses included early-stage calcifying odontogenic cyst, CGCG, and nasopalatine duct cyst. The patient underwent surgery, and all the cysts were resected. The patient was monitored for three years to ensure no recurrence of the disease. The resected lesions were transferred to the pathology laboratory. Figure 4 illustrates the jaw cyst in various profiles. A cranial CT scan confirmed the calcification of the falx cerebri in the coronal and axial views (Figure 5). He was suspected of having Gorlin syndrome due to the presence of two major symptoms of KOT, confirmed by a pathologist, and calcification of the falx cerebri; however, genetic testing was impossible due to its high costs. Radiography of limbs and spine was performed to determine the minor symptoms of Gorlin syndrome, including skeletal deformities; however, no symptoms were found, and radiographs of limbs and spine were normal (Figure 6).
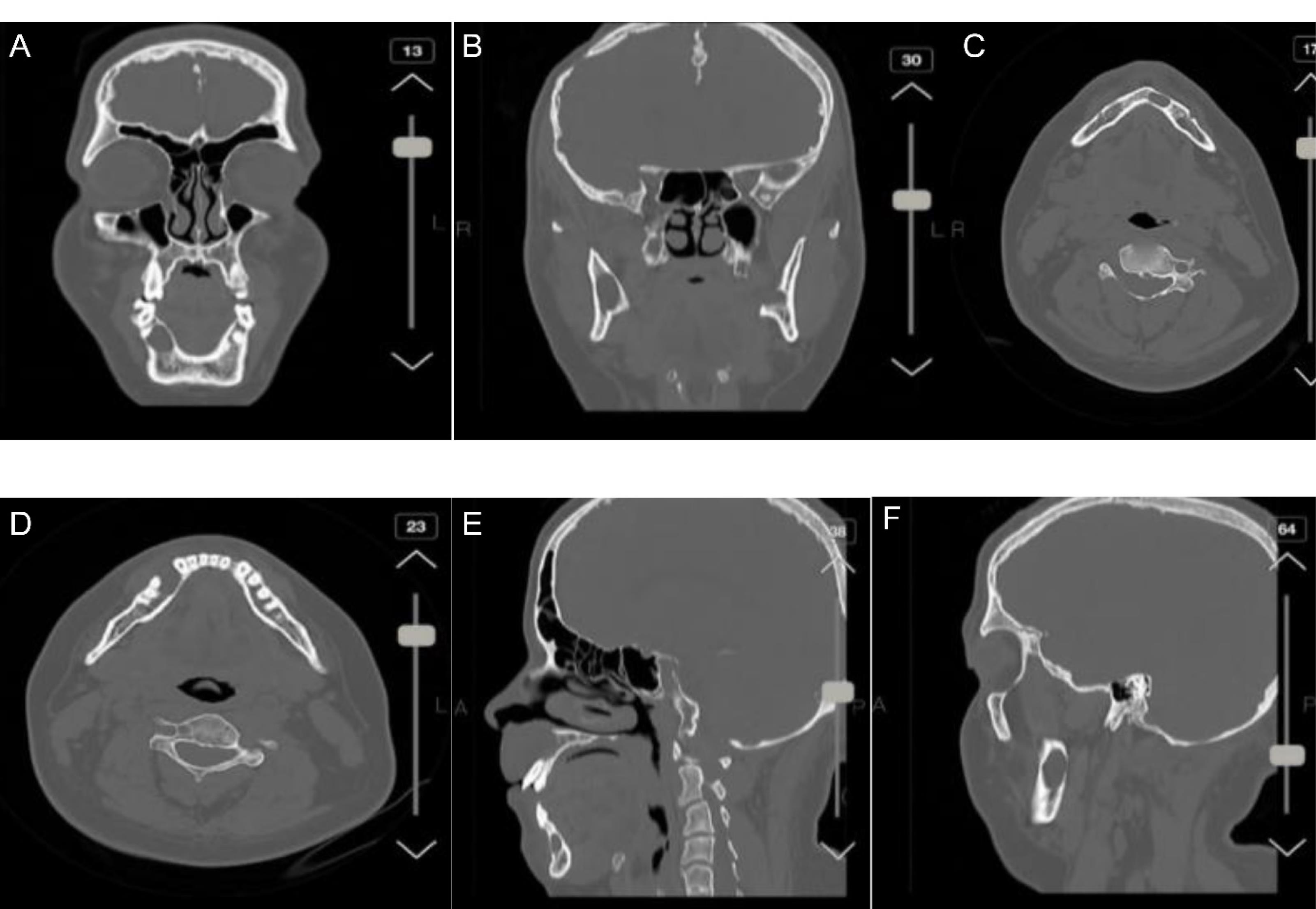
Figure 4.
Jaw Cyst in the Coronal view (A), and in Front of the Mandibular Right Ramus in the Coronal View (B), Cyst Location in the Axial View (C and D), and Sagittal View (E and F)
.
Jaw Cyst in the Coronal view (A), and in Front of the Mandibular Right Ramus in the Coronal View (B), Cyst Location in the Axial View (C and D), and Sagittal View (E and F)
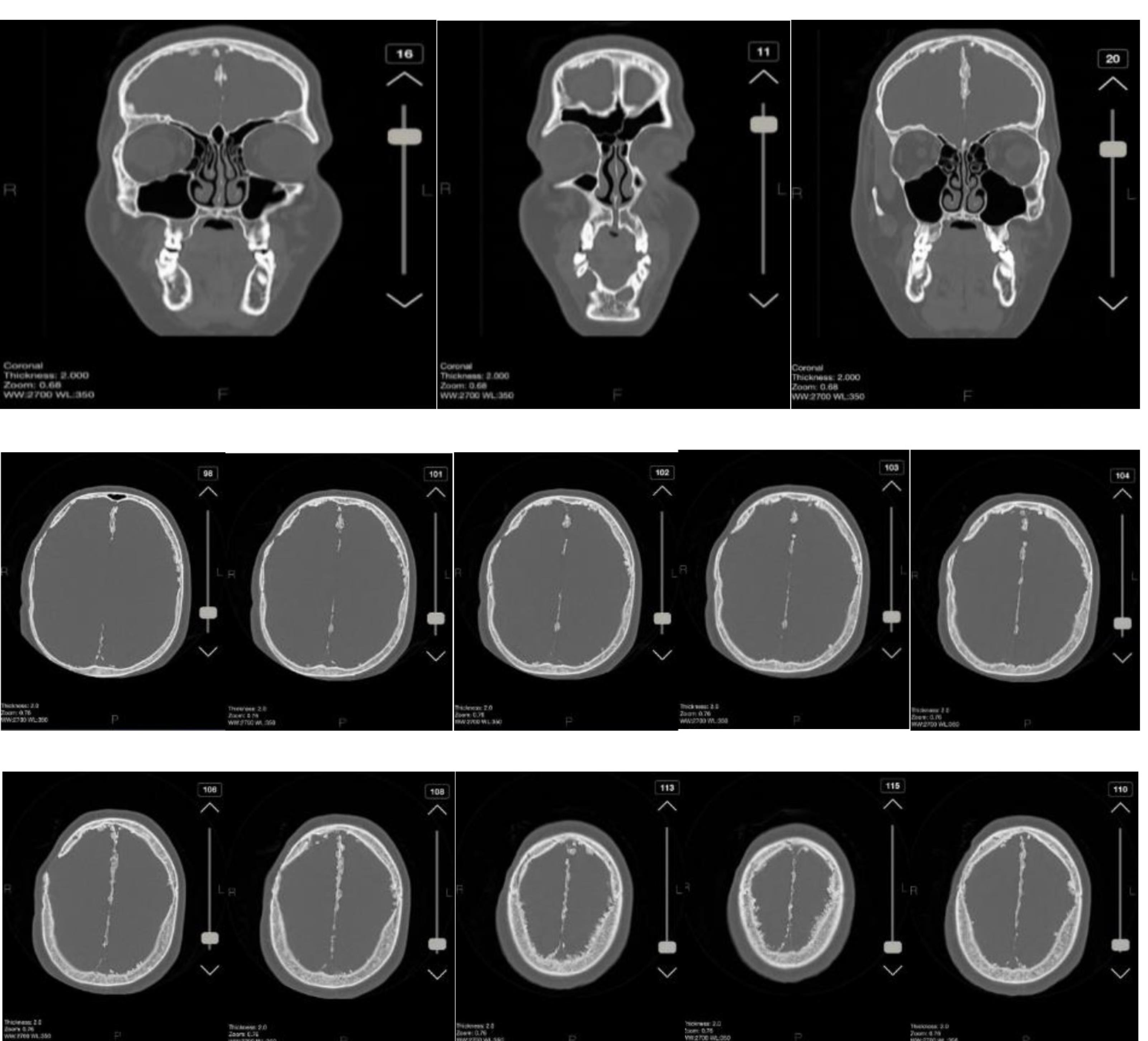
Figure 5.
Calcification of the Falx Cerebri in Coronal and Axial View
.
Calcification of the Falx Cerebri in Coronal and Axial View
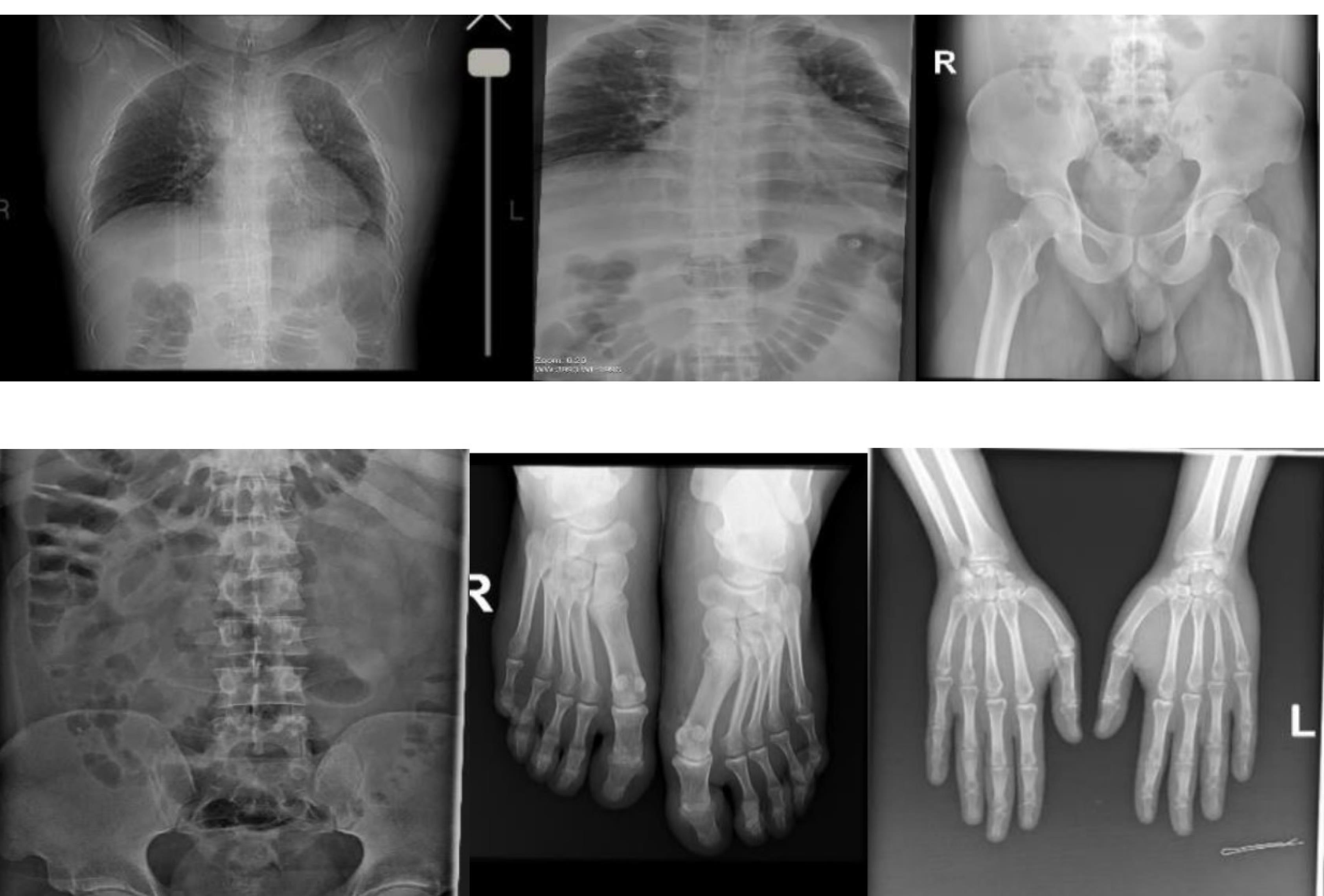
Figure 6.
Computerized Tomography Scan of Limp Showing no Skeletal Abnormalities
.
Computerized Tomography Scan of Limp Showing no Skeletal Abnormalities
Discussion
The present study reported a case with NBCCS and hemophilia A. The presented patient is the first case with hemophilia A diagnosed with NBCCS. Therefore, given the lack of evidence in the literature, to the best of our knowledge, to explain the co-occurrence of these diseases, the relationship between these two disorders remains unclear. NBCCS is a systemic disease characterized by oral and maxillofacial district abnormalities. Hemophilia is introduced as a hereditary condition in which some risk factors lead to the development of oral disease; however, genetic alterations involving the oral structures have not been reported in the disease (7). Recent evidence has confirmed the deep intronic splicing mutations during RNA processing that lead to the formation of cryptic splice sites that compete with the canonical sites. Probably, these mutations play an important role in the pathogenesis of many diseases, including hemophilia A and Gorlin syndrome (8,9). Nonetheless, these data are not enough to explain the possibility of a connection between these two disorders.
NBCCS is mainly characterized by the development of multiple BCCs at an early age. The number of BCCs in the lifetime of patients with NBCCS has been reported to be 500 (2). Harbor aberrations in the hedgehog signaling pathway are reported in NBCCS. This condition occurs due to molecular alterations in the components of a highly conserved hedgehog signaling pathway whose activation leads to tumor cell proliferation caused by the mutation of critical regulatory proteins. Most genetic mutations in NBCCS occur as loss of function mutations in the patched gene (PTCH1) and have been reported in 70% of the cases that fulfill the diagnostic criteria for Gorlin syndrome (10). It should be noted that NBCCS may also arise due to UV exposure in patients not affected by this condition (non-NBCCS patients). In histopathological examination, pearly papules with telangiectasias often appearing on sun-exposed sites are the main clinical features of BCCs. The lesions are histologically indistinguishable from the lesions that occur sporadically. Multiple incidental minute buds of early superficial BCC have been reported in NBCCS; however, these are not specific to NBCCS. BCC lesions in NBCCS often show more than one histologic pattern, including nodular, superficial, and infiltrative subtypes, the most common of which is the nodular type. The characteristics of the nodular histologic pattern of NBCCS include well-circumscribed collections of basaloid epithelial cells with peripheral palisading, retraction artifacts, and frequent mitoses (11).
The major criteria for NBCCS include the calcification of the falx cerebri, the presence of BCC or KOT, palmar or plantar pitting, the presence of medulloblastoma, and a first-degree relative with NBCCS (12). Minor criteria are skeletal and rib malformations, ocular abnormalities, macrocephaly, cleft lip or palate, ovarian or cardiac fibroma, and mesenteric lymph cysts. Molecular confirmation is the criterion for the diagnosis of NBCCS (13).
The presence of KOT and early calcification of the falx cerebri were two major symptoms of NBCCS found in our case. OKC happens as part of the NBCCS; however, it may occur alone and is associated with a genetic mutation at 9q22.3-q31 (the locus for the PTCH1 gene). Therefore, the World Health Organization has reclassified it as a neoplasm rather than an odontogenic cyst (14). PTCH1 plays an important role in tumor suppress; therefore, loss of PTCH1 leads to isolated OKC or NBCCS when occurring as a germline mutation (15). Orthokeratinized epithelium without parakeratosis and basal/parabasal cell palisading is the main differentiation between odontogenic cysts and KOT. Moreover, odontogenic cysts have a low recurrence risk (6,16).
KOTs typically occur in the posterior mandible. Inflammation is often present, and it is the most important reason for the patient’s referral. Cystic neoplasms in OKC may be obscured by inflammation, making the diagnosis more difficult. Although a genetic mutation may be the main cause of the lesions, it can arise coincidentally as well (17). The risk of recurrence has been estimated to be between 10% and 40% for KOTs (16). Radiography is used to detect KOT in an area of lucency often related to the crown of an unerupted tooth. In the presented case, we reported multiple KOTs in both the mandible and maxilla. KOTs in the majority of patients are unilocular (18). However, the present study presented a case with multiloculated KOT.
Skeletal anomalies, cleft lip or palate, macrocephaly, mesenteric lymph cysts, and ocular abnormalities have been introduced as minor manifestations of NBCCS (19). In the present study, two major manifestations of the condition were reported, while no minor manifestation was observable. Although OKC commonly occurs before 20 years of age, in our case, it has presented in adulthood. Dyskeratotic palmar and plantar pitting have been introduced as additional clinical features of NBCCS; however, our case presented none of these symptoms.
Patients with established NBCCS should be under frequent and long-term care. Annual radiologic screening for medulloblastomas and KOTs should be performed in children younger than eight years of age (13). Sun protection is one of the most important preventative care measures (20). In the present case, patients were continually monitored, and no evidence of recurrence was reported three years after surgery. The histologic subtype, size, and location of the tumor are the main factors that should be considered in the selection of a proper treatment plan. In the majority of patients, a large tumor burden has been reported, as observed in our case. Surgery is the most common therapeutic option for NBCCS patients. Topical application of 5-fluorouracil and imiquimod are the other suggested treatment approaches. However, radiotherapy should not be applied to patients with established NBCCS.
Conclusion
To the best of our knowledge, no evidence of the co-existence of NBCCS and hemophilia A exists in the literature, and we have reported it for the first time in the present study. Our case had two major manifestations of established NBCCS, including calcification of the falx cerebri and the presence of KOT; however, no minor manifestations of NBCCS were detectable. Understanding the underlying pathogenesis could help us recognize NBCCS and develop targeted therapeutics.
Authors’ Contribution
Conceptualization: Elham Hadadian Pour, Sirous Risbaf Fakour.
Data curation: Elham Hadadian Pour, Sirous Risbaf Fakour.
Formal analysis: Elham Hadadian Pour.
Funding acquisition: Sirous Risbaf Fakour.
Investigation: Sirous Risbaf Fakour, Amirhosein Haghir.
Methodology: Elham Hadadian Pour, Amirhosein Haghir.
Project administration: Elham Hadadian Pour.
Resources: Amirhosein Haghir.
Software: Elham Hadadian Pour, Sirous Risbaf Fakour.
Supervision: Elham Hadadian Pour.
Validation: Sirous Risbaf Fakour.
Visualization: Sirous Risbaf Fakour.
Writing–original draft: Elham Hadadian Pour.
Writing–review & editing: Elham Hadadian Pour, Amirhosein Haghir.
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
Informed consent was obtained from the patient for publication of this report.
References
- Onodera S, Nakamura Y, Azuma T. Gorlin syndrome: recent advances in genetic testing and molecular and cellular biological research. Int J Mol Sci 2020; 21(20):7559. doi: 10.3390/ijms21207559 [Crossref] [ Google Scholar]
- Al-Jarboua MN, Al-Husayni AH, Al-Mgran M, Al-Omar AF. Gorlin-Goltz syndrome: a case report and literature review. Cureus 2019; 11(1):e3849. doi: 10.7759/cureus.3849 [Crossref] [ Google Scholar]
- Palacios-Álvarez I, González-Sarmiento R, Fernández-López E. Gorlin syndrome. Actas Dermosifiliogr (Engl Ed) 2018; 109(3):207-17. doi: 10.1016/j.ad.2017.07.018 [Crossref] [ Google Scholar]
- Akbari M, Chen H, Guo G, Legan Z, Ghali G. Basal cell nevus syndrome (Gorlin syndrome): genetic insights, diagnostic challenges, and unmet milestones. Pathophysiology 2018; 25(2):77-82. doi: 10.1016/j.pathophys.2017.12.004 [Crossref] [ Google Scholar]
- de Munnik SA, Hoefsloot EH, Roukema J, Schoots J, Knoers NV, Brunner HG. Meier-Gorlin syndrome. Orphanet J Rare Dis 2015; 10:114. doi: 10.1186/s13023-015-0322-x [Crossref] [ Google Scholar]
- Irani S, Dalband M. Orthokeratinized odontogenic cyst: an unusual histopathological presentation. Avicenna J Dent Res 2016; 9(1):e26084. doi: 10.5812/ajdr.26084 [Crossref] [ Google Scholar]
- Fiorillo L, De Stefano R, Cervino G, Crimi S, Bianchi A, Campagna P. Oral and psychological alterations in haemophiliac patients. Biomedicines 2019; 7(2):33. doi: 10.3390/biomedicines7020033 [Crossref] [ Google Scholar]
- Pezeshkpoor B, Zimmer N, Marquardt N, Nanda I, Haaf T, Budde U. Deep intronic ‘mutations’ cause hemophilia A: application of next generation sequencing in patients without detectable mutation in F8 cDNA. J Thromb Haemost 2013; 11(9):1679-87. doi: 10.1111/jth.12339 [Crossref] [ Google Scholar]
- Bholah Z, Smith MJ, Byers HJ, Miles EK, Evans DG, Newman WG. Intronic splicing mutations in PTCH1 cause Gorlin syndrome. Fam Cancer 2014; 13(3):477-80. doi: 10.1007/s10689-014-9712-9 [Crossref] [ Google Scholar]
- Smith MJ, Beetz C, Williams SG, Bhaskar SS, O’Sullivan J, Anderson B. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol 2014; 32(36):4155-61. doi: 10.1200/jco.2014.58.2569 [Crossref] [ Google Scholar]
- Bresler SC, Padwa BL, Granter SR. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol 2016; 10(2):119-24. doi: 10.1007/s12105-016-0706-9 [Crossref] [ Google Scholar]
- Soltaninia O, Ebrahimifard A, Soleimani K. Reconstruction of scalp basal cell carcinoma using pinwheel flap: a case report. Avicenna J Dent Res 2022; 14(2):92-5. doi: 10.34172/ajdr.2022.18 [Crossref] [ Google Scholar]
- Bree AF, Shah MR. Consensus statement from the first international colloquium on basal cell nevus syndrome (BCNS). Am J Med Genet A 2011; 155A(9):2091-7. doi: 10.1002/ajmg.a.34128 [Crossref] [ Google Scholar]
- Barnes L, Eveson J, Reichart P, Sidransky D. World Health Organization Classification of Tumours: Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press; 2005.
- Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer 2008; 8(10):743-54. doi: 10.1038/nrc2503 [Crossref] [ Google Scholar]
- Woo SB. Oral Pathology E-Book: A Comprehensive Atlas and Text. Elsevier Health Sciences; 2016.
- Basile JR, Klene C, Lin YL. Calcifying odontogenic cyst with odontogenic keratocyst: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109(4):e40-5. doi: 10.1016/j.tripleo.2009.12.026 [Crossref] [ Google Scholar]
- da Silva LP, Rolim LS, da Silva LA, Pinto LP, de Souza LB. The recurrence of odontogenic keratocysts in pediatric patients is associated with clinical findings of Gorlin-Goltz syndrome. Med Oral Patol Oral Cir Bucal 2020; 25(1):e56-e60. doi: 10.4317/medoral.23185 [Crossref] [ Google Scholar]
- Thalakoti S, Geller T. Basal cell nevus syndrome or Gorlin syndrome. Handb Clin Neurol 2015; 132:119-28. doi: 10.1016/b978-0-444-62702-5.00008-1 [Crossref] [ Google Scholar]
- Parren LJ, Frank J. Hereditary tumour syndromes featuring basal cell carcinomas. Br J Dermatol 2011; 165(1):30-4. doi: 10.1111/j.1365-2133.2011.10334.x [Crossref] [ Google Scholar]