Avicenna J Dent Res. 15(2):76-80.
doi: 10.34172/ajdr.2023.1652
Case Report
Adenoid Cystic Carcinoma of Parotid Gland with Infratemporal Extension: A Rare Case Report
Mohammad Reza Jamalpour 1  , Tannaz Rasipour 2, *
, Tannaz Rasipour 2, * 
Author information:
1Oral and Maxillofacial Surgery, Department of Oral and Maxillofacial Surgery, School of Dentistry, Dental Implants Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
2Oral and Maxillofacial Surgery, Department of Oral and Maxillofacial Surgery, School of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Adenoid cystic carcinoma (ACC) is a slowly developing malignant tumor of the salivary glands, occurring more commonly in minor salivary glands and rarely in parotid glands. It has the potential of retrograde perineural spread to the adjacent structures and spaces. Here we report a rare case of ACC of the parotid gland extended to the infratemporal fossa with perineural and vascular invasion, reflecting the advanced stage of the disease. After consulting with ENT specialists and radiation oncologists, palliative surgery was performed followed by adjuvant radio/chemo therapy.
Keywords: Adenoid cystic carcinoma, Infratemporal fossa, Parotid gland
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Jamalpour MR, Rasipour T. Adenoid cystic carcinoma of parotid gland with infratemporal extension: A rare case report. Avicenna J Dent Res. 2023; 15(2):76-80. doi:10.34172/ajdr.2023.1652
Background
Adenoid cystic carcinoma (ACC) is a rare malignant tumor that accounts for about 10%-20% of all salivary gland tumors and approximately 1% of all head and neck malignant neoplasms (1,2).
ACC in the head and neck region mainly arises from minor salivary glands and submandibular gland (3). Parotid gland ACCs are relatively rare (4). They can also arise from secretory glands in the nose and paranasal sinuses (PNS), tongue, trachea, nasopharynx, larynx, lacrimal and ceruminous glands, and the external auditory canal (5).
ACC is a slow-growing tumor but has a locally aggressive nature with perineural spread and hematogenous metastasis (6).
The treatment of choice for ACC is radical surgical resection, often with adjuvant radiation and chemotherapy (7,8).
Case Presentation
A 32-year-old man with chief complaints of limited mouth opening, right preauricular swelling, and pain over the past one year was referred to our Oral and Maxillofacial Surgery department of Beasat hospital. He did not have any history of cancer in the head and neck region or drug use.
The examination of the eyes and ears was normal with no evidence of intra-nasal mass or history of epistaxis. The tympanic membranes were intact bilaterally. Cranial nerves I to XII were unimpaired, with no deficiency in the muscles of mastication (VII). There was mild sensory loss in the V3 distribution. Facial mimetic muscles were intact and symmetrical. No evidence of crepitus, pain, or tenderness in temporomandibular Joint (TMJ) was found. Maximum mouth opening was limited (9 mm). In clinical examination, there was an obvious swelling in the right preauricular area that distorted the facial contour. Palpation revealed a well-circumscribed mildly tender mass without any ulceration, pulse, or induration of the overlying soft tissue. Intraoral examination was normal and there was no intraoral extension of the lesion. The parotid papillae were noninflamed bilaterally, with clear saliva secretion from Stensen’s duct. No palpable lymphadenopathy in the submandibular or cervical areas was found.
Sonography of the right parotid and the angle of the mandible revealed a 14 × 30 mm hypoechoic area attached to the medial side of the parotid with extension to the depth of the mandible. Panoramic radiograph confirmed rarefaction in the right mandibular angle (Figure 1). In PNS CT scan with contrast, PNS and nasal mucosa were normal with no lesion. In the neck CT scan with contrast, there was a 26 × 47 mm soft tissue mass in the right infratemporal space with heterogenous contrast enhancement and right parotid invasion. There was no evidence of bone erosion, airway obstruction, or vessel invasion (Figure 2). Skull base cerebellopontine angle (CPA) MRI with contrast demonstrated an ill-defined heterogeneously enhancing mass lesion with a cystic component measuring 43 × 60 mm in the deep portion of the right parotid gland. The lesion extended to the right masticator space and showed close contact with the skull base at the level of the foramen ovale. Internal acoustic meatus had normal width. The cranial nerves CN 6 to CN 9 were intact. No evidence of intracranial or lymph nodes involvement was observed. Brain MRI was normal (Figure 3).
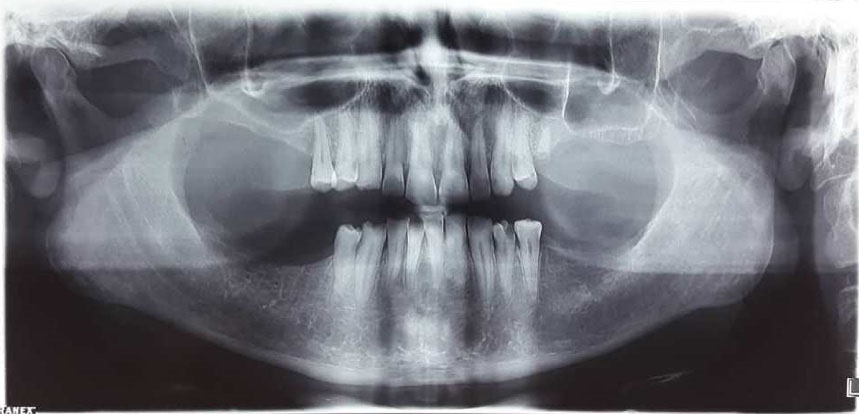
Figure 1.
Preoperative Panoramic Radiograph Showing Rarefaction in the Right Mandibular Angle
.
Preoperative Panoramic Radiograph Showing Rarefaction in the Right Mandibular Angle
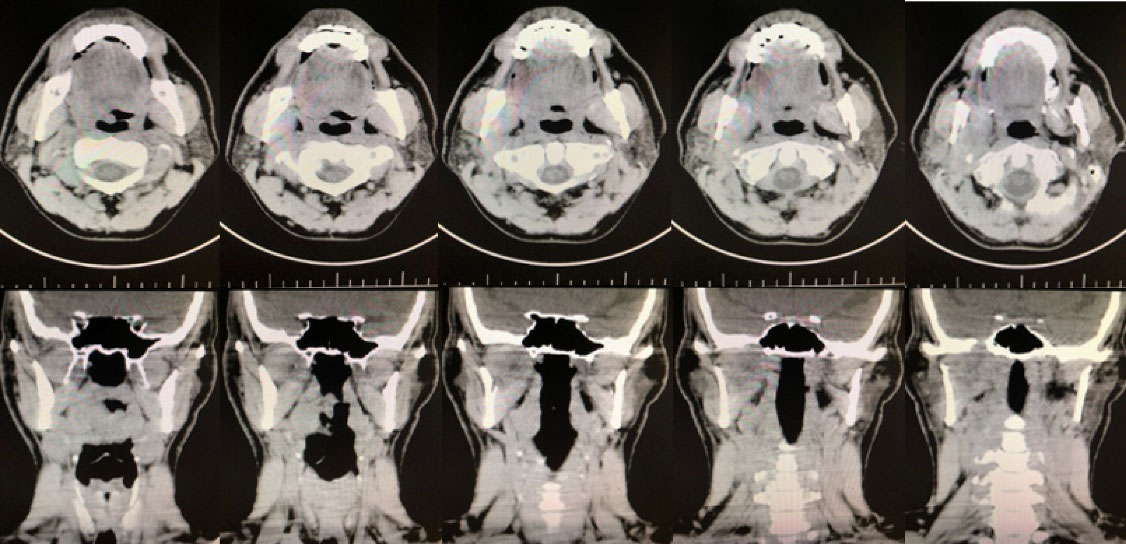
Figure 2.
PNS CT Scan and Neck CT Scan of the Lesion with Contrast in Axial and Coronal Cuts. A 36 × 50 mm soft tissue mass with heterogenous contrast enhancement was found, which originated from deep lobe of the right parotid with extension to the right infratemporal space and right masticator space. Pterygoid muscles, right parapharyngeal fat, and external carotid were involved. Erosion and compressive effect on medial side of right ramus of mandible and greater wing of sphenoid, as well as the involvement and widening of foramen ovale, foramen spinosum, and Meckel’s cave were observed. One enlarged lymph node in right cervical chain was found.
.
PNS CT Scan and Neck CT Scan of the Lesion with Contrast in Axial and Coronal Cuts. A 36 × 50 mm soft tissue mass with heterogenous contrast enhancement was found, which originated from deep lobe of the right parotid with extension to the right infratemporal space and right masticator space. Pterygoid muscles, right parapharyngeal fat, and external carotid were involved. Erosion and compressive effect on medial side of right ramus of mandible and greater wing of sphenoid, as well as the involvement and widening of foramen ovale, foramen spinosum, and Meckel’s cave were observed. One enlarged lymph node in right cervical chain was found.
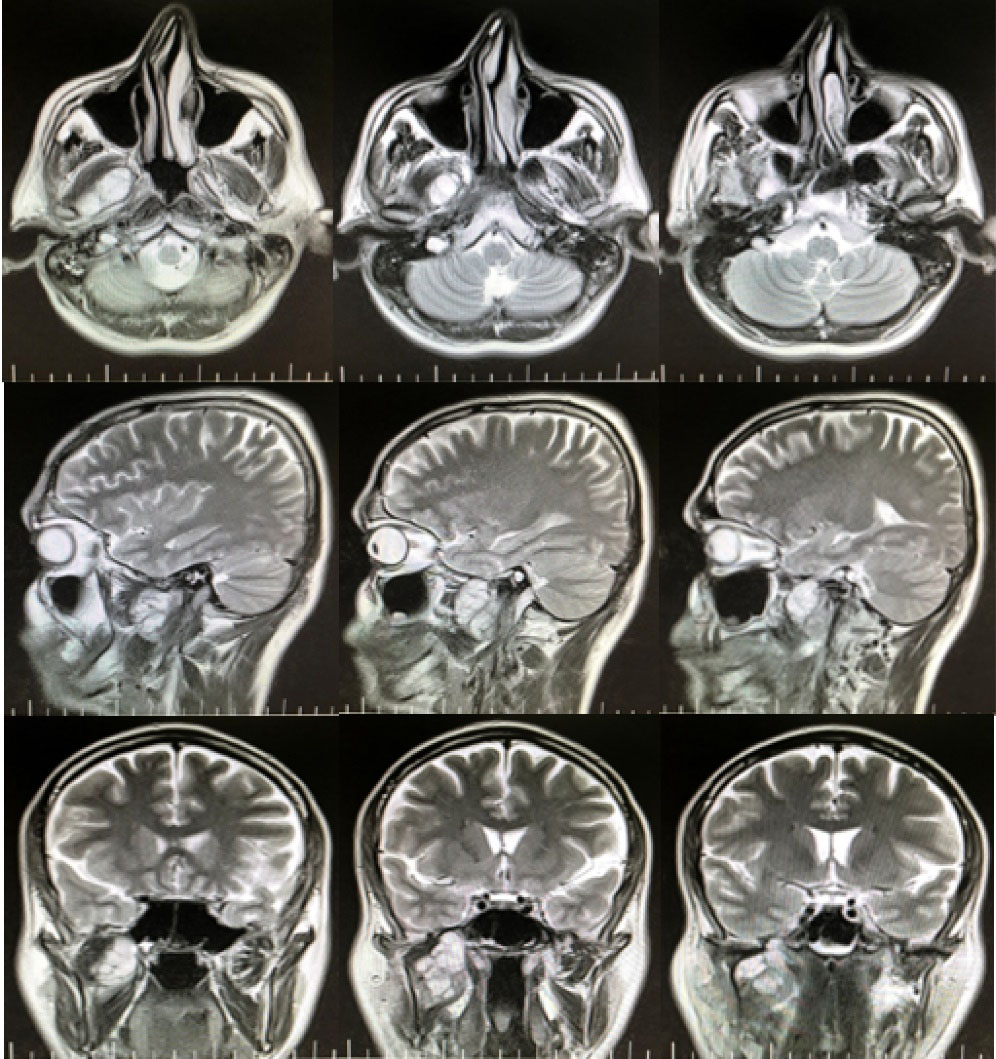
Figure 3.
Skull Base CPA MRI of the Lesion with Contrast in Axial, Sagittal, and Coronal Cuts. An ill-defined heterogeneously enhancing mass lesion with a cystic component was found in deep lobe of the right parotid with extension to the infratemporal space. The lesion extended to foramen ovale in intracranial part but dura seemed intact. Intracranial structures and brain tissue did not seem to be involved. Descending part of facial nerve (fallopian canal/stylomastoid foramen) was involved.
.
Skull Base CPA MRI of the Lesion with Contrast in Axial, Sagittal, and Coronal Cuts. An ill-defined heterogeneously enhancing mass lesion with a cystic component was found in deep lobe of the right parotid with extension to the infratemporal space. The lesion extended to foramen ovale in intracranial part but dura seemed intact. Intracranial structures and brain tissue did not seem to be involved. Descending part of facial nerve (fallopian canal/stylomastoid foramen) was involved.
Routine laboratory test results were within normal limits. Differential diagnoses included pleomorphic adenoma, ACC, schwannoma, lymphoma, juvenile nasopharyngeal angiofibroma, chondrosarcoma, fibrosarcoma, hemangioma, rhabdomyosarcoma, liposarcoma, meningioma, peripheral nerve sheath tumor, neurofibroma, and mucoepidermoid carcinoma.
Considering the advanced stage of the disease and possible malignant differential diagnoses, after consulting with ENT specialists and radiation oncologists, we decide to perform an incisional biopsy and tumor bulk reduction to alleviate trismus and pain and improve future radio/chemo therapy outcomes.
For surgical approach, we used Al-Kayat and Bramley incision to expose the maxillary arch. After zygomatic arch osteotomy, condylotomy with downward repositioning of masseter muscle was performed. Then, coronoidotomy and upward repositioning of the temporalis muscle provided access to the infratemporal lesion. The infratemporal part of the lesion was removed and sent for histopathological examination. Then, condylar reduction and fixation with L plate were performed. For maxillary arch reduction and fixation, we used the coronoid process as free bone graft (Figure 4).
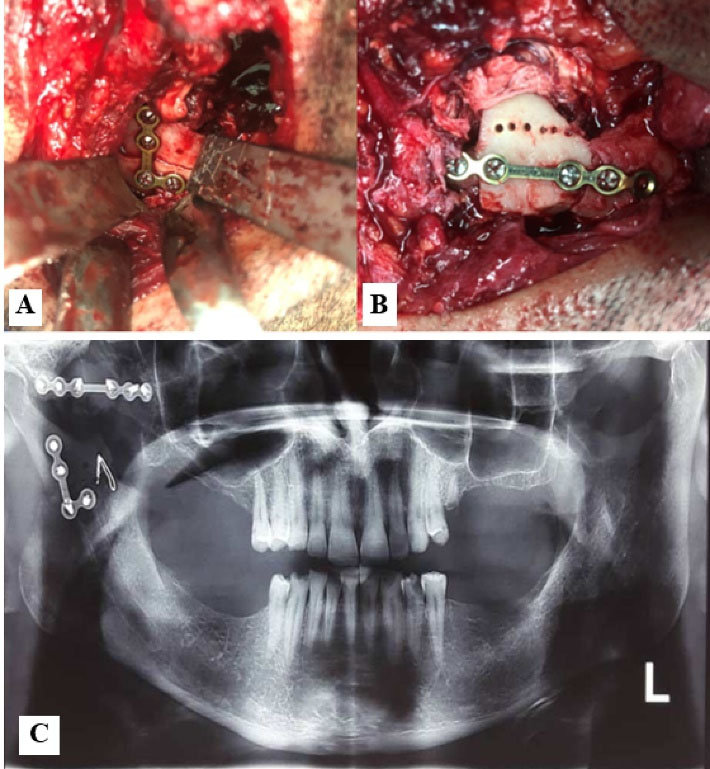
Figure 4.
(A) Condylar reduction and fixation with L plate, (B) Maxillary arch reduction and fixation, coronoid process used as free bone graft, (C) Post-operative panoramic view.
.
(A) Condylar reduction and fixation with L plate, (B) Maxillary arch reduction and fixation, coronoid process used as free bone graft, (C) Post-operative panoramic view.
The pathology report indicated low-grade cribriform and tubular variants of ACC with neural and vascular invasion.
Discussion
The term “adenoid cystic carcinoma” was introduced by Ewing in 1954 (4). The parotid gland ACCs occasionally cause a cranial nerve, particularly the facial nerve, to become paralyzed and manifest as a bulge or solid mass (9). Perineural invasion (22% to 46%) and numerous local recurrences are the major features of this neoplasm (10).
ACC has three histopathologic forms: cribriform, tubular, and solid. The most common pattern is cribriform (11). Any type may have noticeable perineural invasion (12).
Preoperative imaging is necessary for the accurate diagnosis. It mainly aids in determining the anatomical extension of the tumor. The modality of choice for bone invasion is CT scan, while MRI is useful in determining the type of the lesion and assessing its locoregional extent and the cervical lymph nodes and bone marrow infiltration. Considering the potential of retrograde peri-neuronal pathway and a caudal extension of the cervicothoracic passage, it is essential to take skull base imaging (13).
Surgery is the main form of treatment, but adjuvant radiotherapy plays a significant role in more advanced stages and in cases where there are positive margins. A total conservative or a radical parotidectomy is advocated for parotid ACCs though the main intent is to obtain a tumor-free area of at least 1 cm (14). Regional lymph nodes metastasis is uncommon; therefore, neck dissection is typically not necessary (4). Some authors claim that radiotherapy is the only option for treating advanced and non-resectable cancers (15).
Most of the tumors either originating in or extending to the infratemporal fossa are benign, but malignant tumors can also be found. The three cancers that occur most frequently in the infratemporal region are schwannoma, ACC, and juvenile nasopharyngeal angiofibroma (16). Common symptoms of this lesion are facial pain centered over the TMJ and facial numbness. These symptoms were reported to be associated with middle ear effusion and trismus (17). Trismus, facial discomfort, facial hypoesthesia, and neural invasion on MRI are among the imaging or clinical indicators most frequently linked to cancer (16).
According to the literature, the origins of the infratemporal ACCs are the deep lobe of the parotid, ectopic salivary glands (4), external auditory canal (18), ectopic lacrimal glands (19), sinonasal tract (20), and nasopharynx (21).
Conclusions
ACC is a rare malignant tumor of the parotid gland. It can extend to the infratemporal region without obvious clinical symptoms; therefore, early diagnostic tests and imaging studies are important for better treatment prognosis. In case of an infratemporal fossa tumor of occult origin, the deep lobe of the parotid gland can be a potential source.
Authors’ Contribution
Conceptualization: Mohammad Reza Jamalpour.
Data curation: Tannaz Rasipour.
Formal analysis: Mohammad Reza Jamalpour.
Investigation: Mohammad Reza Jamalpour, Tannaz Rasipour.
Methodology: Mohammad Reza Jamalpour, Tannaz Rasipour.
Project administration: Mohammad Reza Jamalpour.
Supervision: Mohammad Reza Jamalpour.
Validation: Mohammad Reza Jamalpour, Tannaz Rasipour.
Visualization: Mohammad Reza Jamalpour, Tannaz Rasipour.
Writing–original draft: Tannaz Rasipour.
Writing–review & editing: Mohammad Reza Jamalpour.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
Written informed consent was obtained from the patent for publication of the report.
References
- Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg 2004; 12(2):127-32. doi: 10.1097/00020840-200404000-00013 [Crossref] [ Google Scholar]
- Vander Poorten VL, Balm AJ, Hilgers FJ, Tan IB, Loftus-Coll BM, Keus RB. Prognostic factors for long term results of the treatment of patients with malignant submandibular gland tumors. Cancer 1999; 85(10):2255-64. doi: 10.1002/(sici)1097-0142(19990515)85:10<2255::aidcncr22>3.3.co;2-4 [Crossref] [ Google Scholar]
- Singaraju M, Singaraju S, Patel S, Sharma S. Adenoid cystic carcinoma: a case report and review of literature. J Oral Maxillofac Pathol 2022; 26(Suppl 1):S26-S9. doi: 10.4103/jomfp.jomfp_458_20 [Crossref] [ Google Scholar]
- Neville BW, Damm DD, Allen C, Chi AC. Oral and Maxillofacial Pathology. Elsevier Health Sciences; 2015. p. 426-28.
- Chen SL, Huang SF, Ho VW, Chuang WY, Chan KC. Clinical characteristics and treatment outcome of adenoid cystic carcinoma in the external auditory canal. Biomed J 2020; 43(2):189-94. doi: 10.1016/j.bj.2019.07.005 [Crossref] [ Google Scholar]
- Yih WY, Kratochvil FJ, Stewart JC. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofac Surg 2005; 63(6):805-10. doi: 10.1016/j.joms.2005.02.021 [Crossref] [ Google Scholar]
- Bjørndal K, Krogdahl A, Therkildsen MH, Overgaard J, Johansen J, Kristensen CA. Salivary gland carcinoma in Denmark 1990-2005: a national study of incidence, site and histology Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol 2011; 47(7):677-82. doi: 10.1016/j.oraloncology.2011.04.020 [Crossref] [ Google Scholar]
- Vander Poorten V, Bradley PJ, Takes RP, Rinaldo A, Woolgar JA, Ferlito A. Diagnosis and management of parotid carcinoma with a special focus on recent advances in molecular biology. Head Neck 2012; 34(3):429-40. doi: 10.1002/hed.21706 [Crossref] [ Google Scholar]
- Barnes L, Eveson JW, Sidransky D, Reichart P. Pathology and Genetics of Head and Neck Tumours. IARC; 2005. p. 221-3.
- Gurney TA, Eisele DW, Weinberg V, Shin E, Lee N. Adenoid cystic carcinoma of the major salivary glands treated with surgery and radiation. Laryngoscope 2005; 115(7):1278-82. doi: 10.1097/01.mlg.0000165381.64157.ad [Crossref] [ Google Scholar]
- Hallacq P, Labrousse F, Roullet B, Orsel S, Bessede JP, Moreau JJ. [Adenoid cystic carcinomas invading the skull base. Apropos of 4 cases and review of the literature]. Neurochirurgie 2001;47(6):542-51. [French].
- Amit M, Binenbaum Y, Trejo-Leider L, Sharma K, Ramer N, Ramer I. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck 2015; 37(7):1038-45. doi: 10.1002/hed.23710 [Crossref] [ Google Scholar]
- Siewert B, Kruskal JB, Kelly D, Sosna J, Kane RA. Utility and safety of ultrasound-guided fine-needle aspiration of salivary gland masses including a cytologist’s review. J Ultrasound Med 2004; 23(6):777-83. doi: 10.7863/jum.2004.23.6.777 [Crossref] [ Google Scholar]
- Marx RE, Stern D. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Hanover Park: Quintessence Publishing Company; 2012. p. 550-3.
- Ouatassi N, Elguerch W, Bensalah A, Maaroufi M, Alami MN. Unusual presentation of parotid gland adenoid cystic carcinoma: a case presentation and literature review. Radiol Case Rep 2022; 17(2):344-9. doi: 10.1016/j.radcr.2021.10.043 [Crossref] [ Google Scholar]
- Lisan Q, Leclerc N, Kania R, Guichard JP, Herman P, Verillaud B. Infratemporal fossa tumors: when to suspect a malignant tumor? A retrospective cohort study of 62 cases. Eur Ann Otorhinolaryngol Head Neck Dis 2018; 135(5):311-4. doi: 10.1016/j.anorl.2018.06.005 [Crossref] [ Google Scholar]
- Shapshay SM, Elber E, Strong MS. Occult tumors of the infratemporal fossa: report of seven cases appearing as preauricular facial pain. Arch Otolaryngol 1976; 102(9):535-8. doi: 10.1001/archotol.1976.00780140067006 [Crossref] [ Google Scholar]
- Jiang X, Jia L, Zhang X, Zhong C, Tang F, Chen X. Clinical experience of 23 cases of adenoid cystic carcinoma of the external auditory canal. Oncol Lett 2020; 20(5):144. doi: 10.3892/ol.2020.12005 [Crossref] [ Google Scholar]
- Nava-Castañeda Á, Kahuam-López N, De La Fuente Díez Y, Velásco YLA, Sánchez-Bonilla FG, Martín F. Primary adenoid cystic carcinoma arising from an ectopic lacrimal gland involving both nasal orbits: a rare clinical entity. Orbit 2021; 40(6):525-8. doi: 10.1080/01676830.2020.1817948 [Crossref] [ Google Scholar]
- Michel G, Joubert M, Delemazure AS, Espitalier F, Durand N, Malard O. Adenoid cystic carcinoma of the paranasal sinuses: retrospective series and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis 2013; 130(5):257-62. doi: 10.1016/j.anorl.2012.09.010 [Crossref] [ Google Scholar]
- Ng BHK, Tang IP. Adenoid cystic carcinoma of the nasopharynx: a case series. Indian J Otolaryngol Head Neck Surg 2019; 71(Suppl 1):731-3. doi: 10.1007/s12070-018-1523-0 [Crossref] [ Google Scholar]