Avicenna J Dent Res. 15(1):18-22.
doi: 10.34172/ajdr.2023.1635
Original Article
Effect of Incorporating Titanium Dioxide Nanoparticles (1%) on Shear Bond Strength of Orthodontic Composites: An In Vitro Study
Melika Firouzmanesh 1  , Abbas Farmany 2, Maryam Farhadian 3, Vahid Mollabashi 1, *
, Abbas Farmany 2, Maryam Farhadian 3, Vahid Mollabashi 1, *
Author information:
1Department of Orthodontics, Dental research center, Dental school, Hamadan University of Medical Sciences, Hamadan, Iran
2Dental research center, Hamadan University of Medical Sciences, Hamadan, Iran
3Department of Biostatistics, School of Public Health and Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Background: Plaque accumulation and bond failure are the drawbacks of fixed orthodontic treatment. Titanium dioxide (TiO2 ) could be added to orthodontic composite as an antimicrobial agent, but it may change its mechanical properties. The aim of this study was to evaluate the mechanical properties of orthodontic composite modified by TiO2 nanoparticles (NPs) after 10000 cycles of thermocycling.
Methods: Overall, 50 intact human premolars (extracted for orthodontic treatment) were used in this study. The orthodontic composite containing TiO2 NPs (1% wt) was prepared and used for the bonding of brackets. The bracket/tooth shear bond strength (SBS) was measured by using a universal testing machine before and after 10000 cycles of thermocycling at 5 and 55° C (dwell time=30 seconds). Eventually, the obtained data were analyzed by Student’s t test with the Excel software (significance level≤0.05).
Results: After thermocycling, the average SBS of TiO2 containing and control group was 11.43±5.18 MPa and 13.46±5.17 MPa, respectively. The difference in the SBS of the two groups after thermocycling was not significant (P=0.7). The SBS of both groups decreased after thermocycling; however, the reduction was lower in the group with TiO2 than in the control group.
Conclusions: TiO2-containing composite can be used as an antimicrobial agent in high risk of caries patients without deteriorating the mechanical properties.
Keywords: Orthodontics, Bonding, Titanium dioxide, Shear strength
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Firouzmanesh M, Mollabashi V, Farmany A, Farhadian M.Effect of incorporating titanium dioxide nanoparticles (1%) on shear bond strength of orthodontic composites: An in vitro study. Avicenna J Dent Res. 2023; 15(1):18-22. doi:10.34172/ajdr.2023.1635
Background
Today, the use of bonding has become a routine process in orthodontic treatment. However, it causes complications such as plaque accumulation and increases the risk of caries (1,2). During orthodontic treatments, oral bacteria colonies/biofilms significantly increase, and the rate of demineralization in people undergoing orthodontic treatment is extremely higher than in other people (2). Recent studies concentrated on reducing microbial accumulations around the brackets, and various methods have already been proposed, including fluoride mouthwash, the use of new-shape brackets that occupy fewer tooth surfaces, and the addition of different antibacterial materials such as titanium dioxide (TiO2), silver nanoparticles (NPs), chitosan, chlorhexidine, and the like to orthodontic composites (3-7). Using dental nanomaterials/nanocomposites has facilitated the production of materials with better bonds and fewer complications (8).
TiO2 NPs are photocatalysts and are used in surface disinfectation. The antimicrobial activity of TiO2 NPs in combination with orthodontic composites on Streptococcus mutansand Streptococcus sanguinis due to the prevalence of these microorganisms in the dental plaque has been investigated in previous research (9). Recent studies have shown the antibacterial effect of composite with TiO2 NPs up to 10% on S. mutans and S. sanguinis (10).
Obviously, the addition of any material to orthodontics composites may change its mechanical properties. Therefore, in addition to having antimicrobial properties, the material must make the least amount of change in the mechanical properties of the composite in an acceptable range. The measurement of bond strength in orthodontics is mainly performed in the form of tensile bond strength and shear bond strength (SBS) methods. Most studies have examined the SBS as the force application, and loading conditions in this method seem to be more close to the clinical conditions of oral environments (11).
Thermocycling is employed to simulate the oral environment in terms of thermal changes. Every 10 000 thermal cycles indicate one year of the clinical operation of a composite (12). Recent studies have investigated the shear strength of orthodontic composites when immersed in water at 37°C under 500 and 5000 thermal cycles (13,14). Furthermore, it was revealed that the immersion of a composite with 0.1% wt TiO2 in water at 37°C for 1 day, 1 month, and 3 months does not affect the SBS of the composites (13).
The performed laboratory studies do not perfectly indicate the long-term performance of a TiO2 composite since 10 000 thermal cycles represent a year of clinical operation of the composite, and orthodontic treatment is likely to take more than a year. This study focused on evaluating the effect of adding 1% wt TiO2 NPs on the SBS of the orthodontic composite after 10 000 cycles at 5 and 55 °C (dwell time = 30 seconds).
Materials and Methods
The required sample size was calculated based on the following equation:
It should be noted that the values required to determine the sample size have been obtained from similar research (13). While the confidence level of this test and the test power were 95% and 90%, the expected difference in the average bond strength was assumed to be µ1-µ2 = 7 units, and the standard deviation was σ = 7. Finally, 20 samples (40 samples in 2 groups) were determined after replacing the minimum sample size required in this study.
In general, 50 healthy extracted human premolars (without cracks or caries) extracted during the orthodontic treatment were collected in this in vitro study. The teeth have been immersed in a 10% formalin solution for two weeks to disinfect (15).
A homogeneous orthodontic composite (Beacepaste, USA) mixture containing 1% wt of TiO2 was prepared in a dark room condition.
Synthesis of TiO2 NPs (Size of Titanium NPs: 3-10 nm)
For this purpose, 7 mL of titanium isopropoxide was slowly added to 9 mL of double-distilled water in the stirring condition for 30 minutes at 40°C. TiO was obtained after the hydrolysis of the alkoxide. After centrifugation and washing with double distilled water and methyl alcohol, the obtained white precipitate was inserted in the oven at 80°C for 12 hours to dry (16). Figure 1 shows the electronic microscopic picture of Titanium Dioxide.
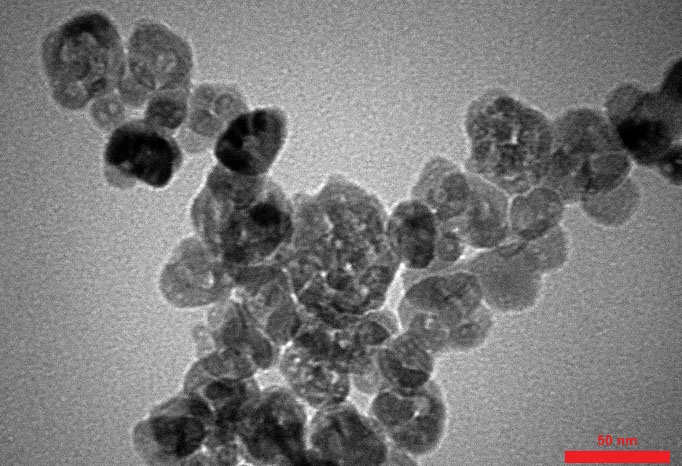
Figure 1.
Electronic Microscopic Picture of Titanium Dioxide
.
Electronic Microscopic Picture of Titanium Dioxide
Procedure
The teeth were vertically mounted on a block of self-cure acrylic. The buccal surface of the crown was polished with fluoride-free pumice paste and then washed and dried. Next, the buccal surface of the teeth was etched with 37% phosphoric acid gel for 20 seconds and washed at a distance of 10-15 cm for 40 seconds. Subsequently, it was dried by air without moisture and fat particles to reveal the fluorosis stains and white spots profile of the enamel.
The teeth were randomly divided into 2 groups as follows:
-
Group 1: After etching and bonding the buccal surface of the tooth, a bracket (Dentaurum, Germany) with a 1% wt TiO2 composite was placed on the buccal surface of the tooth.
-
Group 2: After etching and bonding the buccal surface of the tooth, a bracket (Dentaurum, Germany) with conventional composite was placed on the crown of the tooth.
Excess components were removed with the tip of a sterile scaler. All samples were cured for 20 seconds and 20 seconds from mesial and distal by the light-curing device (Kerr-Germany), respectively (Figure 2). Every 10 minutes, the light cure intensity was checked by a radiometer to ensure that the light intensity of the device was the same for all samples.

Figure 2.
A Bracket Bonded to a Tooth
.
A Bracket Bonded to a Tooth
All the samples were kept in an incubator at 37°C for 24 hours in double-distilled water to complete their polymerization and water absorption. The SBS of 5 samples from each group (10 randomly samples in total) was measured. The remaining samples of groups 1 and 2 were then placed in a DELTA-TP02 thermocycler (Mashhad, Iran) to be subjected to 10 000 thermal cycles at 5-55 °C (dwell time = 30 seconds).
The samples of all groups were placed in a shear strength measuring device (SANTAM -STM-20), and loading was applied at a speed of 1 mm/min (Figures 3 and 4).

Figure 3.
Sample Placed in the Measuring Device to Measure the Shear Bond Strength
.
Sample Placed in the Measuring Device to Measure the Shear Bond Strength
Figure 4.
Shear Bond Strength of One Sample Measured by the Device
.
Shear Bond Strength of One Sample Measured by the Device
The shear strength of the samples was calculated in MPa using the following formula:
In this study, the cross-section area is the base bracket area (12.14 mm2).
Results
Before thermocycling, the average SBS of the TiO2 group (group 1) was 18.33 MPa, and that of the control group (group 2) was 24.09 MPa.
After 10 000 thermal cycles, the average SBS of group 1 was 11.43 ± 5.18 MPa, whereas in group 2, it was 13.46 ± 5.17 MPa (Figure 5). However, the difference in SBS between the two groups was not statistically significant (P = 0.7).
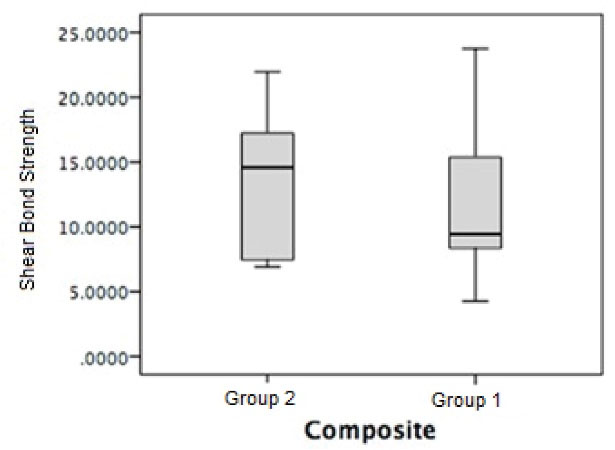
Figure 5.
The Box Plot Shear Bond Strength of the Two Composites
.
The Box Plot Shear Bond Strength of the Two Composites
The SBS of both groups decreased after thermocycling; however, the reduction was lower in the group with TiO2 than in the control group.
Discussion
Recent studies have confirmed that the addition of nanomaterials such as TiO2 NP and the like to orthodontic composites improves their antimicrobial properties while reducing dental white spots and caries. Due to the high prevalence of S. mutans and S. sanguinis in the microbial plaque, the antimicrobial properties of TiO2 NPs in combination with the orthodontic composite on the mentioned organisms have been investigated, and the results demonstrated that the incorporation of these NPs in the composites had a significant effect on reducing the number of microorganisms around the brackets (9,17,18).
A few studies evaluated the effect of NP incorporation on the mechanical properties and SBS of dental brackets prior to recommendation for clinical use. The present study investigated the effect of adding TiO2 NPs to orthodontic composites by simulating one-year clinical use since 10 000 thermal cycles represent a year of the clinical operation of the composite, and orthodontic treatment is likely to take more than a year.
In orthodontics, a firm attachment of the bracket to the tooth is not always desirable. The SBS should be in a way that the bracket stands stable under the forces applied during orthodontics, but an extremely high bond strength causes part of the enamel to peel off during the bracket debond. The minimum SBS required to attach the bracket to the tooth is 6-10 MPa. Increasing the SBS to above 13 MPa causes damage to the enamel and ceramic restorations during the bracket debond.
Sodagar et al compared the SBS of orthodontic adhesives containing 1%, 5%, and 10% by weight of TiO2. There was no statistically significant difference between the control group and the group with 1% and 5% by weight of TiO2, but increasing the concentration of TiO2 to 10% by weight reduced the SBS (19). In compliance with their results, we did not find a significant difference between control and TiO2 (1% wt.) contained group after thermocycling.
Pourhajibagher et al also reported that there were no statistical differences among the SBS of modified orthodontic adhesive containing TiO2 (1%) compared to the control group; however, the control group had higher SBS (20), which is consistent with the result of our study by using the same density of TiO2 incorporation.
According to the results of this study, the addition of TiO2 into composites reduced the average SBS. By simulating one year of the clinical operation (aging) of the composite, the average SBS decreased in both control and TiO2 groups, but this decrease was less considerable in the TiO2 group. The SBSs of both groups after 10 000 thermal cycles are clinically acceptable and do not represent any statistically significant difference.
Behnaz et al exposed composites with and without TiO2 (0.1%) to the aging process for 1, 30, and 90 days. The mean SBS of TiO2-free composites was significantly higher than that of TiO2-containing composites, but the mean SBS did not change significantly over time (13). We used different concentrations of TiO2 and aged it more but reached the same result. Therefore, the addition of TiO2 to the composite up to the threshold has similar behavior.
In the present study, the SBS of the samples was measured before thermocycling (n = 10). The average SBS of the TiO2 (group 1) and control (group 2) groups was 18.33 MPa and 24.09 MPa, respectively. The SBS of both groups decreased after thermocycling; however, the reduction was lower in the group with TiO2 than in the control group.
Reddy et al reported a 30% reduction in SBS by adding 1% by weight of TiO2 to the composite. However, they did not consider the aging of the composites in the mouth and simply compared the composites without simulating the oral environment (21).
Poosti et al demonstrated the anti-caries properties of the addition of TiO2 NPs to orthodontic composites up to 10%. They reported that the addition of 1% TiO2 did not alter the SBS of the composite, which is consistent with our results. They were different in the aging process. However, the SBS of the composites decreased by an increase in the percentage of TiO2 (10).
The differentiation in the NP percentage and methods of aging will affect the interpretation of the results.
Recommendation
Due to the proven anti-bacterial properties of TiO2 and the acceptable SBS of composites with 1% TiO2, the clinical application of 1% TiO2 composites in patients at high risk of caries and, if a cost-efficient protocol is provided, is recommended in all patients. Therefore, further clinical studies in this area are suggested as well.
Conclusions
The TiO2 containing composite can be used as an antimicrobial agent in high risk of caries patients without deteriorating the SBS of bonding.
Authors’ Contribution
Conceptualization: Vahid Mollabashi.
Data curation: Melika Firouzmanesh.
Formal Analysis: Maryam Farhadian.
Funding acquisition: Vahid Mollabashi.
Investigation: Melika Firouzmanesh, Vahid Mollabashi, Abbas Farmany.
Methodology: Vahid Mollabashi, Melika Firouzmanesh, Abbas Farmany.
Project administration: Vahid Mollabashi, Melika Firouzmanesh.
Resources: Vahid Mollabashi- Melika Firouzmanesh- Abbas Farmany.
Supervision: Vahid Mollabashi.
Validation: Vahid Mollabashi.
Visualization: Melika Firouzmanesh.
Writing – original draft: Melika Firouzmanesh.
Writing – review & editing: Vahid Mollabashi, Abbas Farmany, Maryam Farhadian.
Competing Interests
The authors reported no potential conflict of interests relevant to this article.
Ethical Approval
The Research Ethics Committee of Hamadan University of Medical Sciences approved the study (IR.UMSHA.REC.1399.769).
References
- Tufekci E, Dixon JS, Gunsolley JC, Lindauer SJ. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod 2011; 81(2):206-10. doi: 10.2319/051710-262.1 [Crossref] [ Google Scholar]
- Boersma JG, van der Veen MH, Lagerweij MD, Bokhout B, Prahl-Andersen B. Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: influencing factors. Caries Res 2005; 39(1):41-7. doi: 10.1159/000081655 [Crossref] [ Google Scholar]
- Ahn SJ, Lee SJ, Kook JK, Lim BS. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater 2009; 25(2):206-13. doi: 10.1016/j.dental.2008.06.002 [Crossref] [ Google Scholar]
- Felemban NH, Ebrahim MI. The influence of adding modified zirconium oxide-titanium dioxide nano-particles on mechanical properties of orthodontic adhesive: an in vitro study. BMC Oral Health 2017; 17(1):43. doi: 10.1186/s12903-017-0332-2 [Crossref] [ Google Scholar]
- Tanbakuchi B, Bahador A. Nanoparticles in orthodontics: a review article. J Dent Med 2018;31(2):119-33. [Persian].
- Cao B, Wang Y, Li N, Liu B, Zhang Y. Preparation of an orthodontic bracket coated with an nitrogen-doped TiO2-xNy thin film and examination of its antimicrobial performance. Dent Mater J 2013; 32(2):311-6. doi: 10.4012/dmj.2012-155 [Crossref] [ Google Scholar]
- Mirhashemi AH, Bahador A, Kassaee MZ, Daryakenari G, Ahmad-Akhoundi MS, Sodagar A. Antimicrobial effect of nano-zinc oxide and nano-chitosan particles in dental composite used in orthodontics. J Med Bacteriol 2013; 2(3-4):1-10. [ Google Scholar]
- Uysal T, Yagci A, Uysal B, Akdogan G. Are nano-composites and nano-ionomers suitable for orthodontic bracket bonding?. Eur J Orthod 2010; 32(1):78-82. doi: 10.1093/ejo/cjp012 [Crossref] [ Google Scholar]
- Kiomarsi N, Zamani P, Bahador A, Hashemikamangar SS, Pourhajibagher M, Kharazifard MJ. Effect of addition of nano-TiO2, nano-SiO2, and a combination of both, on antimicrobial activity of an orthodontic composite. J Contemp Dent Pract 2020; 21(8):857-62. [ Google Scholar]
- Poosti M, Ramazanzadeh B, Zebarjad M, Javadzadeh P, Naderinasab M, Shakeri MT. Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur J Orthod 2013; 35(5):676-9. doi: 10.1093/ejo/cjs073 [Crossref] [ Google Scholar]
- Ahmed H. Craig’s restorative dental materials, fourteenth edition. Br Dent J 2019; 226(1):9. doi: 10.1038/sj.bdj.2019.29 [Crossref] [ Google Scholar]
- Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent 1999; 27(2):89-99. doi: 10.1016/s0300-5712(98)00037-2 [Crossref] [ Google Scholar]
- Behnaz M, Dalaie K, Mirmohammadsadeghi H, Salehi H, Rakhshan V, Aslani F. Shear bond strength and adhesive remnant index of orthodontic brackets bonded to enamel using adhesive systems mixed with TiO2 nanoparticles. Dental Press J Orthod 2018;23(4):43.e1-43.e7. 10.1590/2177-6709.23.4.43.e1-7.onl.
- Eslamian L, Borzabadi-Farahani A, Mousavi N, Ghasemi A. The effects of various surface treatments on the shear bond strengths of stainless steel brackets to artificially-aged composite restorations. Aust Orthod J 2011; 27(1):28-32. [ Google Scholar]
- Salem-Milani A, Zand V, Asghari-Jafarabadi M, Zakeri-Milani P, Banifatemeh A. The effect of protocol for disinfection of extracted teeth recommended by center for disease control (CDC) on microhardness of enamel and dentin. J Clin Exp Dent 2015; 7(5):e552-6. doi: 10.4317/jced.52280 [Crossref] [ Google Scholar]
- Buraso W, Lachom V, Siriya P, Laokul P. Synthesis of TiO2 nanoparticles via a simple precipitation method and photocatalytic performance. Mater Res Express 2018; 5(11):115003. doi: 10.1088/2053-1591/aadbf0 [Crossref] [ Google Scholar]
- Zarif Najafi H, Azadeh N, Motamedifar M. Evaluation of the preventive effect of composites containing silver and TiO2 nanoparticles on demineralization around orthodontic brackets. J Contemp Dent Pract 2020; 21(8):874-9. [ Google Scholar]
- Farzanegan F, Shahabi M, Ehsan Niazi A, Soleimanpour S, Shafaee H, Rangrazi A. Effect of the addition of Chitosan and TiO2 nanoparticles on antibacterial properties of an orthodontic composite in fixed orthodontic treatment: a randomized clinical trial study. Biomed Phys Eng Express 2021; 7(4):045017. doi: 10.1088/2057-1976/ac0609 [Crossref] [ Google Scholar]
- Sodagar A, Ahmad Akhoundi MS, Bahador A, Farajzadeh Jalali Y, Behzadi Z, Elhaminejad F. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in orthodontics. Dental Press J Orthod 2017; 22(5):67-74. doi: 10.1590/2177-6709.22.5.067-074.oar [Crossref] [ Google Scholar]
- Pourhajibagher M, Sodagar A, Bahador A. An in vitro evaluation of the effects of nanoparticles on shear bond strength and antimicrobial properties of orthodontic adhesives: a systematic review and meta-analysis study. Int Orthod 2020; 18(2):203-13. doi: 10.1016/j.ortho.2020.01.011 [Crossref] [ Google Scholar]
- Reddy AK, Kambalyal PB, Patil SR, Vankhre M, Khan MY, Kumar TR. Comparative evaluation and influence on shear bond strength of incorporating silver, zinc oxide, and titanium dioxide nanoparticles in orthodontic adhesive. J Orthod Sci 2016; 5(4):127-31. doi: 10.4103/2278-0203.192115 [Crossref] [ Google Scholar]