Avicenna J Dent Res. 15(3):87-91.
doi: 10.34172/ajdr.492
Original Article
Antibacterial Activity of Different Artemisia dracunculus Extracts Against Dental Caries-Related Pathogens
Bita Arabestani 1  , Laleh Babaeekhou 1, *
, Laleh Babaeekhou 1, *  , Maryam Ghane 2
, Maryam Ghane 2 
Author information:
1Department of Biology, Islamshahr Branch, Islamic Azad University, Islamshahr, Iran
2Islamshahr Branch, Islamic Azad University, Islamshahr, Iran
Abstract
Background: This study investigated the antibacterial properties of four types of tarragon (Artemisia dracunculus) extracts against two caries contributing bacteria, namely, Streptococcus mutans and Streptococcus sobrinus.
Methods: The extracts of A. dracunculus were prepared using n-hexane, ethyl acetate, methanol, and water solvents. In addition, the diameter of inhibition zones, minimum inhibitory concentrations (MICs), and minimum bactericidal concentrations (MBCs) were determined, and finally, MTT (tetrazolium-based colorimetric assay) was used to analyze the cytotoxic effects of the extracts.
Results: The well-diffusion method showed the antibacterial property of four tested extracts against bacteria. Methanol and water extracts made the highest inhibition zone diameters (P<0.001). This was true for both tested bacteria. The mIC of the methanol, water, n-hexane, and ethyl acetate extracts were 0.78, 1.5, 3.1, and 1.5 mg/mL, as well as 0.78, 0.78, 1.5, and 1.5 mg/mL against S. mutans and S. sobrinus, respectively. The half-maximal inhibitory concentration (IC50) values for methanolic, aqueous, ethyl acetate, and n-hexane extracts were 0.78, 0.78, 1.56, and 3.12 mg/mL, respectively.
Conclusions: The results provided a rational reason for the traditional use of A. dracunculus extracts against anti-caries-related bacteria. The methanolic extract demonstrated better activity, thus methanol can probably extract a wider range of plant compounds with antibacterial effects.
Keywords: S. mutans, S. sobrinus, A. dracunculus, Extract, Methanol
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Arabestani B, Babaeekhou L, Ghane M. Antibacterial activity of different Artemisia dracunculus extracts against dental caries-related pathogens. Avicenna J Dent Res. 2023;15(3):87-91. doi: 10.34172/ajdr.492.
Background
The bacterial plaque has a critical and primary role in the pathogenesis of dental caries. It is highly accepted that mutans streptococci(MS), including Streptococcus mutans and Streptococcus sobrinus, contribute to caries. Thesecommonoral pathogens have been isolated from human dental plaques, and their distribution has been investigated in epidemiological studies (1,2). According to the results of some studies, higher scores of decayed, missing, and filled teeth (DMFT) are reported in the co-presence of MS in mouth microflora (1,3-5). It is reported that 4-6 years old children with MS in plaque samples have higher scores of DMFT compared to those with S. mutans alone (2). This result is confirmed by studies on 6-30 years old individuals and pre-school/school children (1,3). Accordingly, the growth inhibition of both mentioned bacteria can be a rational strategy for caries control. It is shown that natural compounds derived from plants, including the plant extracts of Zingiber officinalis and Polygonum cuspidatum, can inhibit the growth and virulence of S. mutansand S. sobrinus (6-8). The genus Artemisia from the Asteraceae family consists of more than 350 species, among which Artemisia dracunculus is native to Asia and has a wide application in food recipes or salads (9). The aerial parts of A. dracunculus have traditionally been used as antitumor, immunomodulator, antimutagen, and antioxidant, and have antibacterial and antifungal properties (10). Regarding the antimicrobial activity of Artemisia-derived compounds, Juteau et al reported that the essential oil (EO) of A. annua aerial parts can inhibit the growth of Enterococcus hirae, Candida albicans, and Saccharomyces cerevisiae (11). In a similar study, Behbahani et al (12) evaluated the antibacterial and antifungal effects of the A. dracunculus extract against Candida albicans, Streptococcus pyogenes, and Staphylococcus aureus. It is accepted that extraction outcomes can change based on different factors, including solvent polarity. The extraction yield of phenolic and flavonoid contents and the antimicrobial capacity of the extracts greatly depend on solvent polarity (13), and more investigations should be conducted to identify the impact of solvents on the antimicrobial activity of plants. Hence, to the best of our knowledge, this is the first study to investigate and compare the antibacterial activity of four types of A. dracunculus extracts, including methanolic, aqueous, ethyl acetate, and n-hexane extracts against S. mutans and S. sobrinus. The half-maximal inhibitory concentration (IC50) value of each extract was determined in the second part of the study.
Methods
MS Strains
Streptococcus mutansPTCC 1601 and S. sobrinusPTCC 1683 were obtained from the Iranian Research Organization for Science and Technology (IROST). The MS bacteria were cultured an-aerobically on TYCSB agar (Tryptone Yeast Cysteine Sucrose agar with 0.2 U/mL Bacitracin, Merck, Germany) at 37°C for 24 hours.
Plant Extraction
Artemisia dracunculus was prepared from the Medicinal Plants and Drugs Research Institute (MPDR) of Shahid Beheshti University of Iran. The fresh leaf of the plant was dried, and the plant extract was obtained by the maceration method (14). The plant’s names were checked in http://www.theplantlist.org, and genus and species were confirmed by macroscopic and molecular analyses. In this study, four solvents, namely, water, methanol, n-hexane, and ethyl acetate (Merck, Germany) were used for the extraction procedure. Further, 200 g of powdered A. dracunculus leaves were separately dissolved in 150 mL of the solvents. Next, the samples were placed in a shaker incubator for 24 hours (at 30°C). The suspensions were then filtrated using the filter paper (Whatman No. 1) 4 times, and the insoluble parts of suspension were taken accordingly. A vacuum rotary machine (Heidolph, Germany) was applied to remove the solvents from the extracts, and the concentrations of 400 mg/mL were prepared with 1% dimethyl sulfoxide (DMSO, Merck, Germany). Finally, the extracts were sterilized using bacteriological filters and stored in the refrigerator (-30 °C) for microbial tests (14).
Antibacterial Activity
Well Diffusion Methods
All extracts were tested for antimicrobial activity by the well diffusion method (15). For the test, the overnight culture of MS bacteria in the TYCSB broth was employed as bacterial inocula and adjusted to contain 1.5×108 CFU/mLbacterial cells (0.5 McFarland standard) with sterile phosphate-buffered saline (pH: 7.2). Subsequently, 10 μL of the bacterial suspension was spread entirely over Mueller-Hinton II agar (Merck, Germany) plates, and the wholes with 6-8 mm diameter were punched with a sterile Cork borer. Next, 100 μL of 25, 50, and 100 mg/mL of each extract solution was introduced into the wells, and the inhibition zones were measured after incubation at 37°C for 24 hours. In this study, 1% dimethyl sulfoxide (DMSO, Merck, Germany) and 25 μg/mL Ampicillin (AppliChem, Germany) were used as negative and positive controls, respectively.
Minimum Inhibitory Concentrations and Minimum Bactericidal Concentrations of the Extracts
The microdilution method was employed for MIC and MBC evaluation (16). Two-fold serial dilutions of the plant extracts (50-0.18 mg/mL) were prepared in the TYCSB broth. Next, 1% DMSO was added to the medium to increase the solubility of the extracts. Then, 100 μL of the solutions and 2 μL of the bacterial inocula (~1.5×108 CFU/mL) were added to each well (96-well microtiter plates; SPL, South Korea). DMSO-medium and bacteria without extracts were applied as a negative control. After an-aerobically incubation (37 °C for 24 hours), the lowest concentration of the extract with no growth turbidity was considered as the MIC. For MBC determination, 5 μL of each well with no turbidity was transferred on the TYCSB agar and incubated an-aerobically (37°C for 24 hours). The concentration was considered MBC if the growth was decreased by ≥ 99.9%.
Cytotoxicity Assay
Cytotoxicity assessment was performed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (17). RPMI-1640, in addition to 10% fetal bovine serum -streptomycin-penicillin (Gibco, UK), was used as a medium to culture the Vero cells, which were incubated for 24 hours at 37°C in humidified air containing CO2 (5%). After the distribution of the cells in 96-well plates and at the cell density of 2×104 to 2×105 per well, they were incubated for the same above-mentioned time and condition. Thereafter, the extracts at different concentrations (0.39-12.5 mg/mL) were added and the plates were incubated for an additional 24 hours under the same conditions. Afterward, the cells were treated with a 10 μL MTT dye solution (5 mg/mL) for 4 hours (at 37°C). The absorbance was measured (490 nm) after the removal of the MTT dye solution and the addition of 100 μL of DMSO (Merck, Germany) using a microplate reader (ELx808, BioTek, USA). The concentration of extracts with a 50% cell growth inhibition (IC50) was considered cytotoxic. All tests were performed in triplicate, and the mean values were recorded accordingly. Cells treated with 0.1% DMSO were employed as the control group (17).
Statistical Analysis
The assays were performed in triplicate. The mean and standard deviation were calculated, and data were processed for analysis using SPSS software (version 20.0/PC; Chicago, IL, USA). A comparison of values and means of the zones of inhibition was performed by the Tukey HSD test, and a P<0.05 was considered to be statistically significant.
Results
Antibacterial Activity
Well Diffusion Methods
According to the results of the well diffusion, all the studied extracts in the tested concentrations (25, 50, and 100 mg/mL) produced growth inhibition zones against S. mutansand S. sobrinus. For both tested bacteria, methanolic extracts produced a significantly higher mean of the zone of inhibition (P<0.001) compared to ethyl acetate in all tested concentrations (Table 1). There was no significant difference between aqueous and methanolic extracts in the zone of inhibition (P=0.22). The aqueous extract significantly (P<0.001) produced a larger zone in comparison with ethyl acetate and n-hexane in 50 and 100 mg/mL concentrations, and no significant difference was observed between n-hexane and ethyl acetate (P=0.34). The correspondence inhibition zone of these extracts is presented in Table 1.
Table 1.
Zone of Inhibition Means Yielded From Artemisia dracunculus Extract Effects on Streptococcus mutansand Streptococcus sobrinus
Extracts
(mg/mL)
|
Mean ± Standard Deviation (mm)
|
|
Streptococcus mutans
|
Streptococcus sobrinus
|
|
25
|
50
|
100
|
P
Value |
25
|
50
|
100
|
P
Value |
| N-hexane |
21.5±0.5abA |
21.9±0.66bA |
23.03±0.68bA |
0.173 |
21.5±0.4bA |
21.9±0.66bA |
23.03±0.68bA |
0.221 |
| Ethyl acetate |
21.07±0.7bB |
21.97±0.85bAB |
23.57±0.67bA |
0.016 |
21.4±0.6bB |
22±0.89bAB |
23.6±0.56bA |
0.017 |
| Methanol |
23.43±0.61aB |
24.97±0.16aAB |
26.13±0.65aA |
0.009 |
23.4±0.6aB |
25±0.26aB |
27.03±0.55aA |
<0.001 |
| Water |
22.37±0.85abB |
24.93±0.8aA |
26.47±0.5aA |
0.001 |
22.5±0.5abB |
24.03±0.45aB |
26.5±0.36aA |
0.002 |
|
P value |
0.021 |
<0.001 |
<0.001 |
|
0.024 |
<0.001 |
<0.001 |
|
Note. The comparison of the means of the zones of inhibition by Tukey HSD test. Means followed by the same lower-case letters in a column and capital letters on the lines do not significantly differ by the Tukey test (P<0.05). The mean of the zones of inhibition by 100 µL ampicillin (25 µg/mL) as a positive control was 22±0.45. No zone of inhibition was observed in the well containing 100 µL of 1% DMSO as a negative control.
MIC and MBC Values
Table 2 provides the results of the MIC and MBC for all the extracts against S. mutansand S. sobrinus. The results showed that the most antibacterial activity against the two studied bacteria was related to water and methanolic extracts (P<0.001), while the least effect belonged to the n-hexane extract (P<0.001). These results confirmed the finding of the well diffusion test.
Table 2.
MIC and MBC (mg/mL) of Different Extracts of Artemisia dracunculus Against Streptococcus mutansand Streptococcus sobrinus
|
Bacterium
|
Extract
|
MIC
|
MBC
|
|
Artemisia dracunculus
|
N-hexane |
3.1c |
6.2d |
| Ethyl acetate |
1.5 b |
3.1 c |
| Methanol |
0.78 a |
1.5 b |
| Water |
1.5 b |
3.1 c |
|
P value |
<0.001 |
<0.001 |
|
Streptococcus sobrinus
|
N-hexane |
1.5 b |
3.1 c |
| Ethyl acetate |
1.5 b |
3.1 c |
| Methanol |
0.78 a |
1.5 b |
| Water |
0.78 a |
1.5 b |
|
P value |
<0.001 |
<0.001 |
Note. MIC: Minimum inhibitory concentration; MBC: Minimum bactericidal concentration. Values followed by the same lower-case letters in a column do not differ significantly (Tukey HSD test, P<0.05).
Cytotoxic Concentrations
MTT was applied to evaluate the effect of plant extracts on cell viability. The obtained results revealed a dose-dependent reduction of cell viability (from 0.39 to 12.5 mg/mL) by the extracts. IC50 values for methanolic, aqueous, ethyl acetate and n-hexane were 0.78, 0.78, 1.56, and 3.12 mg/mL, respectively. As shown in Figure 1, the most cytotoxic effect against Vero cells is related to the methanolic and aqueous extracts and the least to the n-hexane.
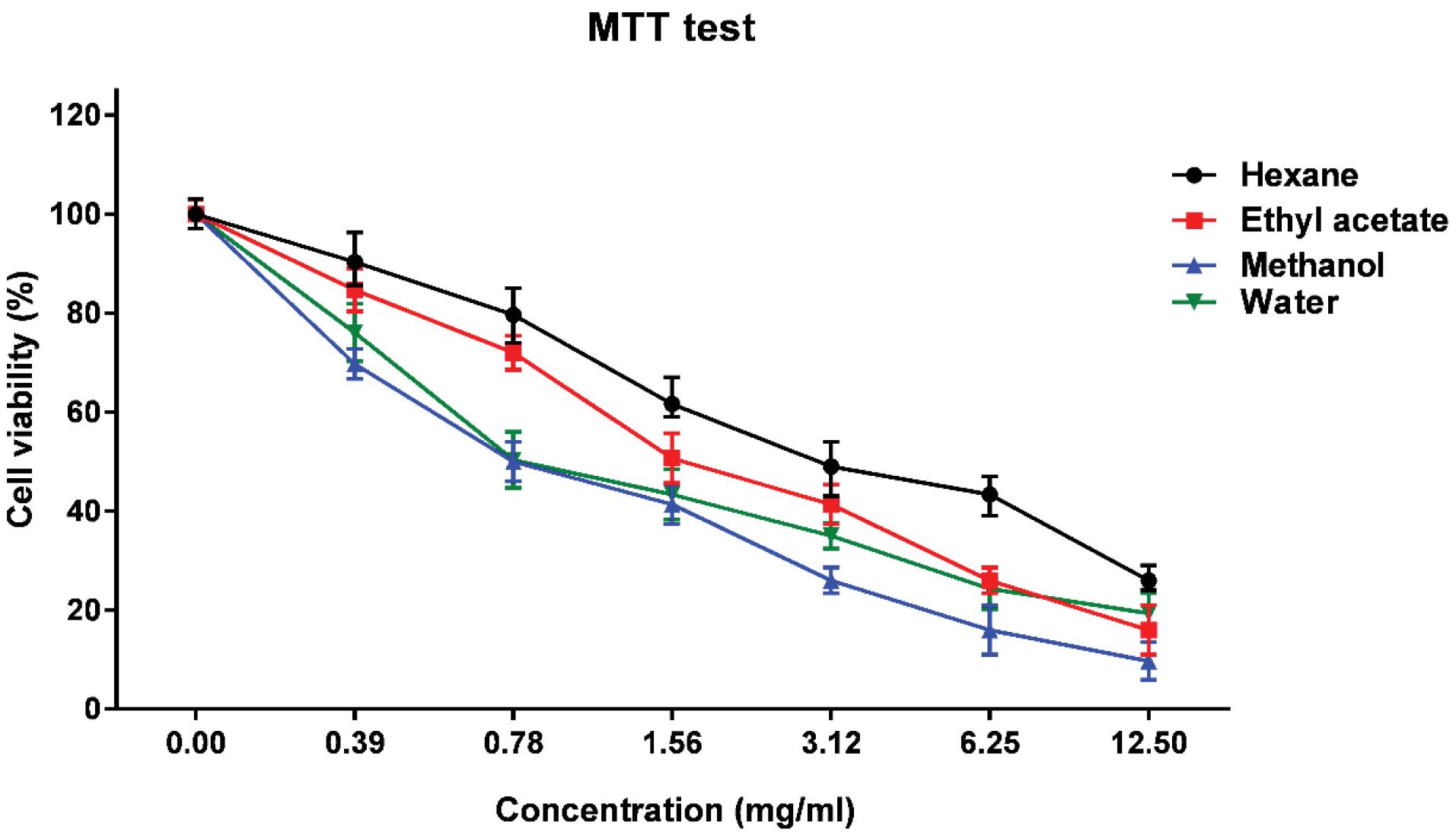
Figure 1.
Viability of the Vero Cells in Different Extract Concentrations of Artemisia dracunculus.
Note.. MTT: Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide.
.
Viability of the Vero Cells in Different Extract Concentrations of Artemisia dracunculus.
Note.. MTT: Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide.
Discussion
According to reports, different Artemisia species have a wide range of biological properties in traditional medicine and have been used as anti-malarial, anthelmintic, antidiabetic agents, and in treating wounds, bronchitis, ulcers, and tuberculosis (18). Higher scores of DMFT are reported in the co-existence of MS in mouth microflora (1,3-5). Thus, the present study focused on evaluating the antibacterial activity of different extracts of A. dracunculus against dental pathogens (S. mutans and S. sobrinus). In this study, the extraction procedure was performed using water, methanol, n-hexane, and ethyl acetate solvents, and antimicrobial assay results confirmed the antibacterial activity for all extract types against S. mutansand S. sobrinus. The water and methanolic extracts with a MIC of 0.78 mg/mL indicated more anti-MS activity, and the lowest antibacterial activity was observed for the n-hexane extract (3.1 mg/mL, P<0.001). The difference in the antibacterial activity of the extracts can be related to different polarities of the solvents which can affect the variety of the extracted compounds (19). Considering that the biological activities of plant extracts are completely associated with their components, the extraction solvent type can directly affect the extract’s activities (20). Oliveira et al focused on Corylus avellana and evaluated the effects of solvents with different polarities on the outcome of the extraction. They revealed that they could increase the number of extracted phenoliccompounds and improve the antioxidant and antibacterial activities of the extracts by changing the type of the solvent(21). Principally, the influencing factors on the biological activity of the plant extract are the methods of drying and extraction and the type of the solvent (20). Our study results on A. dracunculus (tarragon) demonstrated that among the tested solvents, water and methanol were better options for the extraction of compounds with antimicrobial properties.
Erdogrul et al (22) examined the antibacterial activities of methanol, ethyl acetate, acetone, and chloroform extracts from four plant species against 12 bacterial species and concluded that the most antimicrobial property in Artemisia is related to the ethyl acetate extract. The results of the mentioned study are not in agreement with those of our study, and this may be related to the extraction procedure, the tested bacteria, and the type and number of various components of the plant extracts. Previous studies declared that A. dracunculus is mainly composed of phenolic (e.g., carvacrol, γ- and α-terpinene) and flavonoid contents, and it is well accepted that extracts and EOs rich in terpinene and phenolic components can represent noticeable antimicrobial activities (20). Likewise, Raeisiet al (23) investigated the antibacterial effects of the A. dracunculus EO on Escherichia coli and S. aureus. In this study, the observed MIC for E. coli and S. aureus were 2.5 and 1.25 mg/mL, respectively. They further reported that all the EO concentrations for each tested bacterium reduced bacterial count in cheese samples compared to the control (23). Similarly, Mohammadi et al (24) confirmed the anti-quorum sensing and anti-biofilm activities of the A. dracunculus EO on S. aureus and Salmonella enterica serovar Typhimurium.
The safety of plant extracts toward eukaryotic cells should be evaluated since the results of studies on these extracts can be used for complementary development to be consumed by humans. The IC50 of various concentrations of A. dracunculus extracts on the eukaryotic Vero cells was investigated in this study. IC50 values for the tested extract were 0.78 mg/mL for methanolic and aqueous, as well as 1.56 and 3.12 mg/mL for ethyl acetate and n-hexane extracts, respectively. To the best of our knowledge, there is no such report that could highlight the comparative cytotoxic effect of various A. dracunculus extracts. In their study, Motamedifar et al (25) compared the anti-S. mutansactivity ofPeganum Harmala L. with 0.2% chlorhexidine, and then investigated the cytotoxicity of the ethanolic extract of theplanton Vero cells by MTT. The concentrations of more than 0.5 mg/mL caused over 50% of cell destruction. However, the anti-S. mutans activity of the studied plant was lower compared to A. dracunculus.
Conclusions
The results of this study confirmed the strong anti-S. mutans/S. sobrinus property of all types of A. dracunculus extracts. However, considering higher inhibition zone diameters and lower MIC/MBC values of the methanolic and aqueous extracts, these solvents can extract more effective components from the plant. MTT results showed the viability of the Vero cells after exposure to all extract types in bacteriostatic concentrations. Compounds with synergistic activities can also be used to decrease the MIC of the extracts. Thus, A. dracunculus extracts are potential candidates for further analysis and use in the development of herbal medicinal compounds against dental caries.
Author’s Contribution
Conceptualization: Laleh Babaeekhou.
Data curation: Laleh Babaeekhou, Maryam Ghane.
Formal analysis: Laleh Babaeekhou, Maryam Ghane.
Funding acquisition: Laleh Babaeekhou, Maryam Ghane.
Investigation: Laleh Babaeekhou, Maryam Ghane.
Methodology: Laleh Babaeekhou, Maryam Ghane.
Project administration: Bita Arabestani, Laleh Babaeekhou, Maryam Ghane.
Resources: Laleh Babaeekhou, Maryam Ghane.
Software: Laleh Babaeekhou, Maryam Ghane.
Supervision: Laleh Babaeekhou.
Validation: Laleh Babaeekhou.
Visualization: Laleh Babaeekhou, Maryam Ghane.
Writing–original draft: Laleh Babaeekhou.
Writing–review & editing: Laleh Babaeekhou.
Competing Interests
None.
Ethical Approval
Not applicable.
Funding
None.
References
- Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J Med Microbiol 2005; 54(Pt 7):661-5. doi: 10.1099/jmm.0.46069-0 [Crossref] [ Google Scholar]
- Babaeekhou L, Abouie Mehrizi A, Ghane M. Streptococcus mutans, sugar consumption, and oral hygiene: which one has more effect on decayed, missing, and filled teeth (DMFT) score in Iranian adults?. Dent Res J (Isfahan) 2020; 17(2):134-41. [ Google Scholar]
- Okada M, Kawamura M, Oda Y, Yasuda R, Kojima T, Kurihara H. Caries prevalence associated with Streptococcus mutans and Streptococcus sobrinus in Japanese schoolchildren. Int J Paediatr Dent 2012; 22(5):342-8. doi: 10.1111/j.1365-263X.2011.01203.x [Crossref] [ Google Scholar]
- Oda Y, Hayashi F, Okada M. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in patients with intellectual disabilities. BMC Oral Health 2015; 15:102. doi: 10.1186/s12903-015-0087-6 [Crossref] [ Google Scholar]
- Nanda J, Sachdev V, Sandhu M, Deep-Singh-Nanda K. Correlation between dental caries experience and mutans streptococci counts using saliva and plaque as microbial risk indicators in 3-8 year old children A cross Sectional study. J Clin Exp Dent 2015; 7(1):e114-8. doi: 10.4317/jced.51814 [Crossref] [ Google Scholar]
- Babaeekhou L, Ghane M. Antimicrobial activity of ginger on cariogenic bacteria: molecular networking and molecular docking analyses. J Biomol Struct Dyn 2021; 39(6):2164-75. doi: 10.1080/07391102.2020.1745283 [Crossref] [ Google Scholar]
- Song JH, Kim SK, Chang KW, Han SK, Yi HK, Jeon JG. In vitro inhibitory effects of Polygonum cuspidatum on bacterial viability and virulence factors of Streptococcus mutans and Streptococcus sobrinus. Arch Oral Biol 2006; 51(12):1131-40. doi: 10.1016/j.archoralbio.2006.06.011 [Crossref] [ Google Scholar]
- Lee SH. Antimicrobial effects of herbal extracts on Streptococcus mutans and normal oral streptococci. J Microbiol 2013; 51(4):484-9. doi: 10.1007/s12275-013-3312-5 [Crossref] [ Google Scholar]
- Bora KS, Sharma A. The genus Artemisia: a comprehensive review. Pharm Biol 2011; 49(1):101-9. doi: 10.3109/13880209.2010.497815 [Crossref] [ Google Scholar]
- Lee SH, Lee MY, Kang HM, Han DC, Son KH, Yang DC. Anti-tumor activity of the farnesyl-protein transferase inhibitors arteminolides, isolated from Artemisa. Bioorg Med Chem 2003; 11(21):4545-9. doi: 10.1016/j.bmc.2003.08.008 [Crossref] [ Google Scholar]
- Juteau F, Masotti V, Bessière JM, Dherbomez M, Viano J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia 2002; 73(6):532-5. doi: 10.1016/s0367-326x(02)00175-2 [Crossref] [ Google Scholar]
- Alizadeh Behbahani B, Shahidi F, Tabatabaei Yazdi F, Mortazavi SA, Mohebbi M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J Food Meas Charact 2017; 11(2):847-63. doi: 10.1007/s11694-016-9456-3 [Crossref] [ Google Scholar]
- Vuong QV, Hirun S, Roach PD, Bowyer MC, Phillips PA, Scarlett CJ. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J Herb Med 2013; 3(3):104-11. doi: 10.1016/j.hermed.2013.04.004 [Crossref] [ Google Scholar]
- Azwanida NN. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants 2015; 4(3):196. doi: 10.4172/2167-0412.1000196 [Crossref] [ Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 2016; 6(2):71-9. doi: 10.1016/j.jpha.2015.11.005 [Crossref] [ Google Scholar]
- Solmaz G, Ozen F, Ekinci Y, Bird PS, Korachi M. Inhibitory and disruptive effects of shiitake mushroom (Lentinula edodes) essential oil extract on oral biofilms. Jundishapur J Microbiol 2013; 6(9):e9058. doi: 10.5812/jjm.9058 [Crossref] [ Google Scholar]
- Sadeghi I, Yousefzadi M, Behmanesh M, Sharifi M, Moradi A. In vitro cytotoxic and antimicrobial activity of essential oil from Satureja intermedia. Iran Red Crescent Med J 2013; 15(1):70-4. doi: 10.5812/ircmj.4989 [Crossref] [ Google Scholar]
- Zhao GR, Xiang ZJ, Ye TX, Yuan YJ, Guo ZX. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem 2006; 99(4):767-74. doi: 10.1016/j.foodchem.2005.09.002 [Crossref] [ Google Scholar]
- Thongson C, Davidson PM, Mahakarnchanakul W, Weiss J. Antimicrobial activity of ultrasound-assisted solvent-extracted spices. Lett Appl Microbiol 2004; 39(5):401-6. doi: 10.1111/j.1472-765X.2004.01605.x [Crossref] [ Google Scholar]
- Rajabian A, Hassanzadeh Khayyat M, Emami SA, Tayarani-Najaran Z, Rahimzadeh Oskooie R, Asili J. Phytochemical evaluation and antioxidant activity of essential oil, and aqueous and organic extracts of Artemisia dracunculus. Jundishapur J Nat Pharm Prod 2017; 12(1):e32325. doi: 10.5812/jjnpp.32325 [Crossref] [ Google Scholar]
- Oliveira I, Sousa A, Morais JS, Ferreira ICFR, Bento A, Estevinho L. Chemical composition, and antioxidant and antimicrobial activities of three hazelnut (Corylus avellana L) cultivars. Food Chem Toxicol 2008; 46(5):1801-7. doi: 10.1016/j.fct.2008.01.026 [Crossref] [ Google Scholar]
- Erdogrul ÖT. Antibacterial activities of some plant extracts used in folk medicine. Pharm Biol 2002; 40(4):269-73. doi: 10.1076/phbi.40.4.269.8474 [Crossref] [ Google Scholar]
- Raeisi M, Tajik H, Razavi RS, Maham M, Moradi M, Hajimohammadi B. Essential oil of tarragon (Artemisia dracunculus) antibacterial activity on Staphylococcus aureus and Escherichia coli in culture media and Iranian white cheese. Iran J Microbiol 2012; 4(1):30-4. [ Google Scholar]
- Mohammadi Pelarti S, Karimi Zarehshuran L, Babaeekhou L, Ghane M. Antibacterial, anti-biofilm and anti-quorum sensing activities of Artemisia dracunculus essential oil (EO): a study against Salmonella enterica serovar Typhimurium and Staphylococcus aureus. Arch Microbiol 2021; 203(4):1529-37. doi: 10.1007/s00203-020-02138-w [Crossref] [ Google Scholar]
- Motamedifar M, Khosropanah H, Dabiri S. Antimicrobial activity of Peganum harmala L on Streptococcus mutans compared to 02% chlorhexidine. J Dent (Shiraz) 2016; 17(3):213-8. [ Google Scholar]