Avicenna J Dent Res. 13(2):52-56.
doi: 10.34172/ajdr.2021.10
Original Article
Frequency Distribution of Gingival Biotype and Related Factors in an Adult Population of Isfahan
Sima Kiani 1, *  , Saeedeh khalesi 2
, Saeedeh khalesi 2  , Jaber Yaghini 2, Fatemeh Azad 3
, Jaber Yaghini 2, Fatemeh Azad 3
Author information:
1Assistant Professor, Dental Implants Research Center, Department of Periodontics, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran.
2Associate Professor, Dental Implants Research Center, Department of Periodontics, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran.
3Dentist, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran.
Abstract
Background: Gingival biotype can be influenced by genetic factors, tooth-related factors and biological issues. This study aimed to determine the biotype of facial gingival and related factors.
Methods: In this study, 300 patients (128 males and 172 females) with a mean age of 36.2 ± 13.27 were selected by simple random sampling. Patients’ characteristics including age, gender, smoking, dental and keratinized gingival anatomy and oral hygiene parameters were recorded and their associations with gingival biotype were investigated using Transparency method. Collected data were analyzed by SPSS24 using t test, Mann-Whitney, ANOVA, and Pearson correlation coefficient. The P<0.05 was considered significant.
Results: Frequency of thin gingival biotype was higher than that of thick gingival biotype. There was a significant relationship between gingival biotype of upper central incisors areas and age (P < 0.001), vibratory brushing (P=0.019) and keratinized gingival width (P=0.021). There was also a significant relationship between the gingival biotype of lower central incisor area and gender (P=0.036), vibratory brushing (P=0.010), vertical brushing (P=0.009) and keratinized gingival width (P=0.011). Moreover, a significant direct relationship was discovered between Gingival biotype of upper and lower central incisors areas. No relationship was found between frequency and duration of brushing, dental flossing, plaque index, tooth shape, and smoking with gingival biotype (P> 0.005).
Conclusions: Gingival biotype was associated with age, gender and keratinized gingival width, as well as some brushing characteristics such as the brushing method.
Keywords: Gingival biotype, Keratinized tissue, Oral hygiene
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Khalesi S, Kiani S, Yaghini J, Azad F. Frequency Distribution of Gingival Biotype and Related Factors in an Adult Population of Isfahan. Avicenna J Dent Res. 2021;13(2):52-56. doi: 10.34172/ajdr.2021.10.
Background
Highlights
-
Gingival biotype was associated with age, gender and keratinized gingival width
-
Frequency of thin gingival biotype was higher than that of thick gingival biotype.
Determining the dimensions of different parts of the masticatory mucosa, especially the gingival biotype, has been considered by periodontists in recent years (1,2). Healthy gingiva is essential for protecting teeth and maintaining their position (3). The term “gingival biotype” is used to describe the thickness of the gingiva in the facial and palatal dimensions. Gingiva with thinner biotypes are more prone to gingival resorption. Gingival thickness plays an important role in wound healing and flap management during reconstructive surgery (4). It is necessary to know the characteristics of the gingiva – especially gingival thickness, before restorative and prosthetic treatments (5,6). Furthermore, gingival biotype has crucial implications for regenerative, implant and restorative treatments (7-10). Transplant hematopoiesis may be stopped by a very thick graft tissue or may contract the mucosa due to the thin graft tissue (6). Previous studies have shown that patients with thin biotype experience more gingival recession during non-surgical treatments (11). These patients are also more prone to connective tissue loss and epithelial damage and need non-traumatic treatments, as well as special oral hygiene methods (12). However, the thick biotype is more resistant to physical trauma or gingival resorption, and it facilitates better tissue management (13). Since the gingival biotype may be influenced by other factors such as genetic and racial factors (14), this study attempted to investigate the frequency of gingival biotype and its relationship with sociological factors, related anatomical structures, and oral health habits in adult population in Isfahan, Iran.
Materials and Methods
In this cross-sectional study, the prevalence of gingival biotype and its relationship with related anatomical structures, oral hygiene habits, and some other environmental factors related to adult patients referring to Isfahan Dental Centers were investigated. First, the treatment centers were selected by cluster sampling and the patients were included in the study by simple sampling and based on the inclusion criteria. Out of 14 districts of Isfahan Municipality, 5 districts (north, south, east, west and center) and one clinic in each district were randomly selected based on the list of medical system. In each clinic, 30 women (10 women aged 19-24 years, 10 women aged 25-44 years, and 10 women aged 45-65 years) and 30 men (10 men aged 19-24 years, 10 men aged 25-44 years, and 10 men aged 45-65 years) were examined and, finally, 300 patients were included in the study. Patients aged 19-65 with at least 20 teeth, gingival index less than 2 (15), and periodontal pocket depth less than 3 mm were included in the study. Patients with a history of systemic disease, pregnant women and patients taking periodontal drugs such as cyclosporine A and calcium channel blockers and the like were excluded from the study. Also, patients with subgingival restoration, veneers or fixed and removable orthodontic appliances in the studied teeth, hyperplasia or history of periodontal surgery, and crowding or malalignment teeth were all excluded from the study.
Then the research plan was explained to each patient and if s/he was satisfied with the plan, a record of her/his sociological characteristics including age, gender and smoking habits was completed. The desired characteristics including brushing frequency, duration of brushing, brushing method, and dental flossing frequency were also recorded. Next, the Plaque index (16) was calculated. The dimensions of the tooth, including its length (in the midfacial from the incisal edge to the gingival zenith), its width (the widest part of the tooth at the buccal contact points) and the ratio of these two were recorded. When the ratio of tooth (width to crown length) was less than 43%, it was described as triangular tooth. When this ratio was more than 57%, it was defined as square and if the ratio between 43%-57%, it was defined as rectangular (17). Then gingival biotype and keratinized gingival thickness were measured with Williams probe (Joya, Pakistan) in the upper and lower left central teeth areas. Data were analyzed by SPSS 24 using t test, Mann-Whitney, ANOVA and Pearson correlation coefficient. The P<0.05 was considered significant.
Results
In this study, 300 patients aged 19 to 65 were included where 128 (42.7%) were male, and 172 (57.3%) were female. Regarding the upper left central region, 168 patients had thin biotypes and 132 patients had thick biotypes. Concerning the lower left central region, 207 patients had thin biotypes and 93 patients had thick biotypes (Figure 1). According to T-Test statistical analysis, there was a significant relationship between age and gingival biotype of the upper and lower left central teeth regions (P<0.001). Therefore, thick gingival biotype had been increased along with aging.
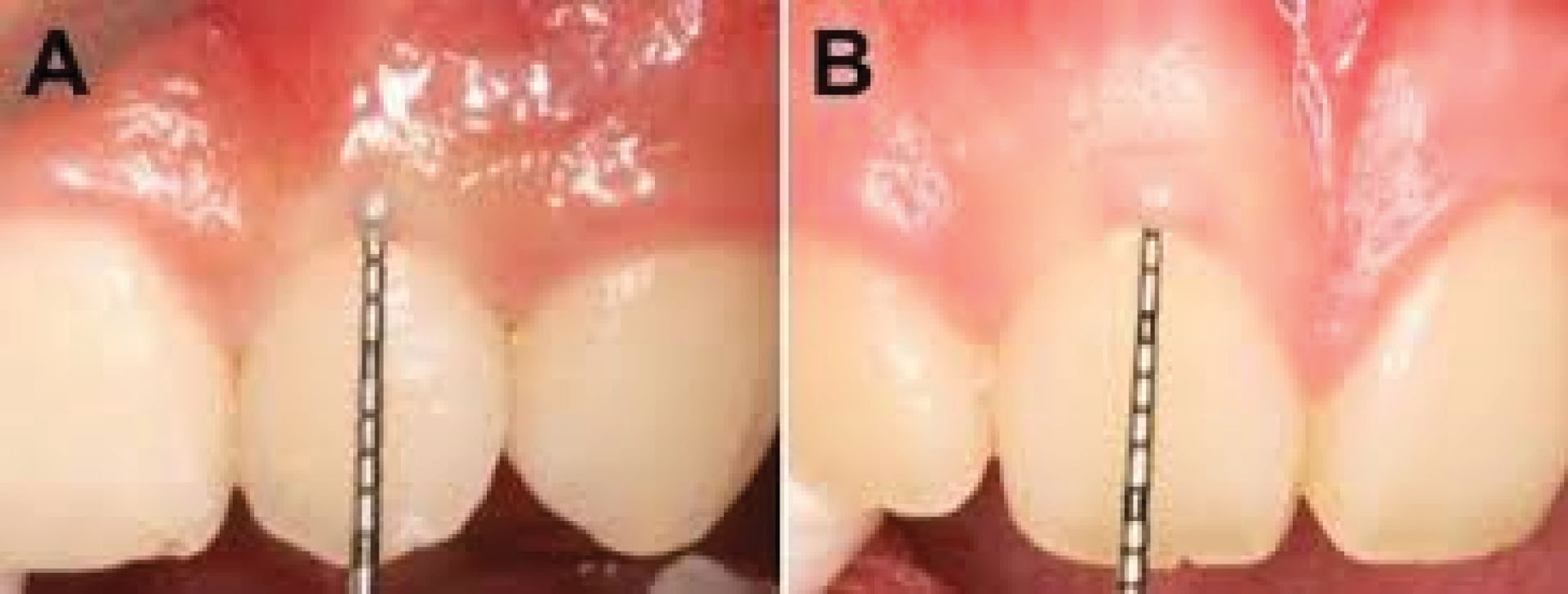
Figure 1.
(A) Thick Gingival Biotype, (B) Thin Gingival Biotype.
.
(A) Thick Gingival Biotype, (B) Thin Gingival Biotype.
According to the chi-square test, there was no significant relationship between gender and the gingival biotype of the upper left central tooth area (P = 0.585). However, a significant relationship was reported between the gingival biotype of the lower left central tooth area and gender (P = 0.036). Given these results, majority of men had thicker biotypes than females (Table 1).
Table 1.
Frequency of the Studied Variables and its Relationship With the Biotype of Upper and Lower Left Central Teeth
|
Variables
|
|
No. (%)
|
P
value
Association with upper gingival biotype
|
P
value
Association with lower gingival biotype
|
| Brushing frequency |
Never |
16 (5.4) |
0.597 |
0.895 |
| Once a day |
201 (67.2) |
| Twice a day |
70 (23.4) |
| More than twice a day |
12 (4) |
| Duration of brushing |
< 30 seconds |
3 (1.1) |
0.94 |
0.812 |
| Between 30 seconds to 1 minute |
26 (9.2) |
| Between 1 and 2 minutes |
152 (53.5) |
| > 2 minutes |
102 (36.3) |
| Brushing method |
Horizontal |
283 (99.6) |
0.368 |
0.502 |
| Hinged |
28 (9.9) |
0.313 |
0.114 |
| Trembling |
14 (4.9) |
0.019 |
0.01 |
| Vertical |
182 (63.9%) |
0.334 |
0.009 |
| Dental flossing frequency |
Never |
140 (46.8) |
0.075 |
0.235 |
| Sometimes |
68 (22.7) |
| Once a day |
53 (17.7) |
| More than twice a day |
38 (12.7) |
| Maxillary plaque index |
0 |
96 (32) |
0.953 |
- |
| 1 |
117 (39) |
| 2 |
74 (24.7) |
| 3 |
13 (4.3) |
| Mandibular plaque index |
0 |
80 (26.7) |
- |
0.17 |
| 1 |
138 (46) |
| 2 |
68 (22.7) |
| 3 |
14 (4.7) |
| Shape of upper central incisor |
Triangle |
0 (0) |
0.551 |
- |
| Rectangle |
1 (0.3) |
| Square |
298 (99.7) |
| Shape of lower central incisor |
Triangle |
11 (3.7) |
- |
0.735 |
| Rectangle |
62 (20.7) |
| Square |
226 (75.6) |
The mean width of keratinized gingiva was 6.8 mm in the upper central tooth region, whereas it was 4.33 mm in the lower central tooth region. Following the logistic regression analysis, significant differences were reported between keratinized gingiva and gingival biotype of upper left central tooth region (P = 0.021), and between keratinized gingiva and gingival biotype of lower left central tooth region (P = 0.011). With an increase in keratinized gingiva, therefore, thin biotype in the upper left central area and thick biotype in the lower left central area were observed.
Majority of patients (67.2%) used a toothbrush once a day and the duration of brushing for most of them (52.5%) was between one to two minutes. Patients mostly used horizontal brushing (99.6%) and vertical brushing (63.9%). Table 1 shows the relationship between different brushing methods and gingival biotype. Our study results revealed no significant relationship between dental flossing frequency and gingival biotype (P > 0.05). Many patients (46.8%) had never used dental floss, although no significant difference was found between them and other patients in terms of gingival biotype.
Majority of patients had plaque index 1 in the maxilla and mandible. According to logistic regression statistical analysis, no significant relationship was observed between the plaque index of the jaw and gingival biotype (Table 1).
Most patients had square upper central teeth (99.7%) as well as square lower central teeth (75.6%), and there was no significant relationship between tooth shape and gingival biotype (Table 1).
In this study, 17 patients had a smoking history. According to Mann-Whitney test, no significant difference was identified between cigarette pack index and gingival biotype of upper left central tooth area (P = 0.79), or between cigarette pack index and gingival biotype of lower left central tooth area (P = 0.814). Following chi-square test, the relationship between gingival biotype of upper and lower central teeth areas proved to be significant (P <0.001).
Discussion
This study, carried out in Isfahan, aimed to determine the relationship of age, gender, brushing characteristics, dental flossing, plaque index, keratinized gingiva, as well as tooth shape and smoking with gingival biotype of upper and lower left central teeth areas among a group of adults.
The gingival biotype was revealed to be significantly thinner in younger patients, which was probably due to increased gingival keratinization at older ages. This result is consistent with the results of a study by Mousavi et al conducted in Golestan University of Medical Sciences (18). The result is also in the line with the results of studies by Müller et al and Wara-aswapati et al (19,20). However, it differs from the results of a study done in 2005 by Vandana and Savitha (21). The discrepancy discovered in Vandana and Savitha’s study may be due to the insufficient sample size (n = 32) and a different method applied for determining the gingival biotype, namely UNC-15 probe using anesthesia at the mucoconjunctival junction to the margin of the gingival margin. Eger et al have shown that there is no difference in gingival biotype among age groups (14). Contrary to the results of our study, Shariatmadar Ahmadi et al reported that the gingiva in younger age group (24-26 years) was significantly thicker than the gingiva in older age group (24-38 years) (22). However, a smaller sample size in the study of Shariatmadar Ahmadi et al may reduce the validity of its results.
In the present study, it was found that the biotype of the lower left central gingiva was thinner in females than in males, which is consistent with the findings of a study by Bhat (23). The results of our study are also consistent with the findings of studies by Müller et al (19) and Vandana and Savitha (21) and García-Cortés et al (2). In Shariatmadar Ahmadi and colleagues’ study, gingival biotype was reported to be thicker in males (22). However, in our study, no significant relationship was found between the upper central gingival biotype and gender. In this regard, our study is in line with the study of Sadeghi et al who only examined the anterior teeth of the maxilla (24).
Our study also demonstrated that the frequency of thick gingival biotypes in the maxilla was higher than the mandible, which is consistent with the results of Müller and colleagues’ study (19) but is inconsistent with Vandana’s study results (21). The finding reported in Vandana and Savitha’s study may relate to its insufficient sample size (32 people), different method of gingival biotype determination, and the differences in population race (21).
In this study, it was found that in people with thinner gingival biotypes, the average width of keratinized gingiva was lower in the mandible but higher in the maxilla. The studies by García-Cortés et al and Mousavi et al have shown that the average width of keratinized gingiva is also lower in people with thin gingival biotype (2,18). Following Sadeghi and colleagues’ study, it has been indicated that there is a significant relationship between gingival biotype of maxillary central teeth area and the amount of keratinized gingiva (24).
Regarding the relationship between gingival thickness and tooth shape, no significant relationship was discovered in our study. Müller et al have reported that rectangular teeth have less gingival thickness than square teeth (19). It seems that the difference in gingival biotype according to the type of tooth and the location of the dental arch is associated with the difference in the shape and size of the teeth, or is related to the buccolingual position of the teeth. It may be also due to the amount of bony protrusion in the alveolar ridge area at the facial aspect (21). In earlier studies, it has been reported that gingival biotype is not associated with the crown shape (4-7). However, García-Cortés et al have reported that square teeth are associated with thin biotypes (2).
Eger et al have showed that there is no significant relationship between the ratio of tooth width to length and gingival biotype. The method adopted for measuring gingival biotype in their study was using ultrasonic devices, which is one of the strengths of their study (14).
Dental plaque control is largely influenced by the method and skill of individuals in brushing (25). According to the results of our study, the vibratory brushing method was directly and significantly associated with the thin gingival biotype in the upper and lower central teeth regions, while the hinged brushing method was associated with the thinness of the lower central tooth gingival biotype. Scanty studies have compared the results regarding brushing and gingival biotype methods so far. Smutkeeree et al showed that both rubbing and bass methods significantly reduced plaque and gingival index (26). In another study by Heidari et al, it was demonstrated that the rubbing method was more effective than the rotational method in reducing plaque (25).
In the present study, no significant relationship was found between different brushing times and gingival biotype. In a study by Tsamtsouris et al, no significant difference was discovered between brushing time and the amount of plaque removal (27). In our study, no significant relationship was found between brushing frequency and gingival biotype. In the study by García-Cortés et al (2), no correlation was determined between gingival biotype and the frequency of brushing, flossing, and smoking, which is consistent with our study results.
Conclusions
Reporting our study results, the gingival biotype was found to be associated with age, gender and keratinized gingival width, as well as with some brushing characteristics such as the method of brushing. However, further studies are required to explore the relationship between gingival biotype and oral health factors.
Study Limitations and Suggestions
Few studies have ever examined the gingival biotype in Iran and, therefore, more studies with larger sample sizes are needed to confirm the results from our study. Due to the prevalence of COVID-19 pandemic, the number of the patients willing to participate in clinical trials has been decreased dramatically.
Conflict of Interest Disclosures
The authors have no conflict of interests.
Acknowledgments
This study was supported by Isfahan University of Medical Sciences Research Grant # 399467. This research also was supported by Dental Materials Research Center of Isfahan University of Medical Sciences.
Ethical Statement
The present study was approved by Dental Implant Research Center of Isfahan University of Medical School. All the procedures were conducted under the Declaration of guidelines of the Ethics Committee of the Isfahan University of Medical Science (IR.MUI.RESEARCH.REC, 1399.472)
Authors’ Contribution
SK and SKh and FA: Concepts, design, definition of intellectual content,Literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, and manuscript preparation, manuscript editing, manuscript review; JY and FA:Concepts, design, clinical studies, experimental studies,manuscript editing, manuscript review; and a guarantor. All authors read and approved the manuscript.
References
- Do JH, Takei HH, Carranza FA. Periodontal examination and diagnosis. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds. Newman and Carranza’s Clinical Periodontology. 13th ed. Elsevier Saunders; 2019. p. 389-90.
- García-Cortés JO, Loyola-Rodríguez JP, Monárrez-Espino J. Gingival biotypes in Mexican students aged 17-19 years old and their associated anatomic structures, socio-demographic and dietary factors. J Oral Sci 2019; 61(1):156-63. doi: 10.2334/josnusd.17-0370 [Crossref] [ Google Scholar]
- Fiorellini JP, Kim D, Chang YC. Anatomy, structure, and function of the periodontiom. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds. Newman and Carranza’s Clinical Periodontology. 13th ed. Elsevier Saunders; 2019. p. 19.
- Rajpoot N, Nayak A, Nayak R, Bankur PK. Evaluation of variation in the palatal gingival biotypes using an ultrasound device. J Clin Diagn Res 2015; 9(3):ZC56-60. doi: 10.7860/jcdr/2015/12041.5724 [Crossref] [ Google Scholar]
- Nagaraj KR, Savadi RC, Savadi AR, Prashanth Reddy GT, Srilakshmi J, Dayalan M. Gingival biotype - Prosthodontic perspective. J Indian Prosthodont Soc 2010; 10(1):27-30. doi: 10.1007/s13191-010-0011-3 [Crossref] [ Google Scholar]
- Pontoriero R, Carnevale G. Surgical crown lengthening: a 12-month clinical wound healing study. J Periodontol 2001; 72(7):841-8. doi: 10.1902/jop.2001.72.7.841 [Crossref] [ Google Scholar]
- Anderegg CR, Metzler DG, Nicoll BK. Gingiva thickness in guided tissue regeneration and associated recession at facial furcation defects. J Periodontol 1995; 66(5):397-402. doi: 10.1902/jop.1995.66.5.397 [Crossref] [ Google Scholar]
- Baldi C, Pini-Prato G, Pagliaro U, Nieri M, Saletta D, Muzzi L. Coronally advanced flap procedure for root coverage Is flap thickness a relevant predictor to achieve root coverage? a 19-case series. J Periodontol 1999; 70(9):1077-84. doi: 10.1902/jop.1999.70.9.1077 [Crossref] [ Google Scholar]
- Kois JC. Predictable single tooth peri-implant esthetics: five diagnostic keys. Compend Contin Educ Dent 2001; 22(3):199-206. [ Google Scholar]
- Alzoubi IA, Hammad MM, Abu Alhaija ES. Periodontal parameters in different dentofacial vertical patterns. Angle Orthod 2008; 78(6):1006-14. doi: 10.2319/092807-462.1 [Crossref] [ Google Scholar]
- Manjunath RG, Rana A, Sarkar A. Gingival biotype assessment in a healthy periodontium: transgingival probing method. J Clin Diagn Res 2015; 9(5):ZC66-9. doi: 10.7860/jcdr/2015/13759.5956 [Crossref] [ Google Scholar]
- Fischer KR, Richter T, Kebschull M, Petersen N, Fickl S. On the relationship between gingival biotypes and gingival thickness in young Caucasians. Clin Oral Implants Res 2015; 26(8):865-9. doi: 10.1111/clr.12356 [Crossref] [ Google Scholar]
- Singh J, Rathod VJ, Rao PR, Patil AA, Langade DG, Singh RK. Correlation of gingival thickness with gingival width, probing depth, and papillary fill in maxillary anterior teeth in students of a dental college in Navi Mumbai. Contemp Clin Dent 2016; 7(4):535-8. doi: 10.4103/0976-237x.194117 [Crossref] [ Google Scholar]
- Eger T, Müller HP, Heinecke A. Ultrasonic determination of gingival thickness Subject variation and influence of tooth type and clinical features. J Clin Periodontol 1996; 23(9):839-45. doi: 10.1111/j.1600-051x.1996.tb00621.x [Crossref] [ Google Scholar]
- Alkan Ö, Kaya Y, Alkan EA, Keskin S, Cochran DL. Assessment of gingival biotype and keratinized gingival width of maxillary anterior region in individuals with different types of malocclusion. Turk J Orthod 2018; 31(1):13-20. doi: 10.5152/TurkJOrthod.2018.17028 [Crossref] [ Google Scholar]
- Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol 1967; 38(6):610-6. doi: 10.1902/jop.1967.38.6.610 [Crossref] [ Google Scholar]
- Gobbato L, Tsukiyama T, Levi PA Jr, Griffin TJ, Weisgold AS. Analysis of the shapes of maxillary central incisors in a Caucasian population. Int J Periodontics Restorative Dent 2012; 32(1):69-78. [ Google Scholar]
- Mousavi T, Fakhari E, Roshandel G. Investigation of the Relationship of Gingival Biotype with the Width of Keratinized Gingiva, Depth of Probe, and Height of Papilla in Patients Referring to the Dental School at Golestan University of Medical Sciences. J Mashhad Dent Sch 2020; 44(3):271-8. doi: 10.22038/jmds.2020.46409.1878 [Crossref] [ Google Scholar]
- Müller HP, Heinecke A, Schaller N, Eger T. Masticatory mucosa in subjects with different periodontal phenotypes. J Clin Periodontol 2000; 27(9):621-6. doi: 10.1034/j.1600-051x.2000.027009621.x [Crossref] [ Google Scholar]
- Wara-aswapati N, Pitiphat W, Chandrapho N, Rattanayatikul C, Karimbux N. Thickness of palatal masticatory mucosa associated with age. J Periodontol 2001; 72(10):1407-12. doi: 10.1902/jop.2001.72.10.1407 [Crossref] [ Google Scholar]
- Vandana KL, Savitha B. Thickness of gingiva in association with age, gender and dental arch location. J Clin Periodontol 2005; 32(7):828-30. doi: 10.1111/j.1600-051X.2005.00757.x [Crossref] [ Google Scholar]
- Shariatmadar Ahmadi R, Yousefi Y, Shahravi F, Gholamali pour N. Evaluation of gingival thickness and related factors in a group of Iranian adults. J Res Dent Sci 2012; 9(2):105-10. [ Google Scholar]
- Bhat V, Shetty S. Prevalence of different gingival biotypes in individuals with varying forms of maxillary central incisors: a survey. J Dent Implant 2013; 3(2):116-21. doi: 10.4103/0974-6781.118888 [Crossref] [ Google Scholar]
- Sadeghi R, Sarlati F, Kalantari S. Relationship between crown forms and periodontium biotype. J Res Dent Sci 2011; 8(1):1-8. [ Google Scholar]
- Heidari K, Mojahedi M, Seyed Moalemi Z, Golshahi H. Comparison of scrub and roll brushing techniques in controlling dental plaque in 8-11 year-old children. J Isfahan Dent Sch 2012; 8(4):322-9. [ Google Scholar]
- Smutkeeree A, Rojlakkanawong N, Yimcharoen V. A 6-month comparison of toothbrushing efficacy between the horizontal Scrub and modified Bass methods in visually impaired students. Int J Paediatr Dent 2011; 21(4):278-83. doi: 10.1111/j.1365-263X.2011.01120.x [Crossref] [ Google Scholar]
- Tsamtsouris A, White GE, Clark ER. The effect of instruction and supervised toothbrushing on the reduction of dental plaque in kindergarten children. ASDC J Dent Child 1979; 46(3):204-9. [ Google Scholar]