Avicenna J Dent Res. 13(2):67-71.
doi: 10.34172/ajdr.2021.13
Original Article
Effect of Chlorhexidine Containing Fluoride on Dental Plaque, Gingivitis, and Tooth Discoloration: A Randomized Clinical Trial
Fatemeh Taghizadeh 1  , Elham Fakhari 2, *
, Elham Fakhari 2, * 
Author information:
1Dental student, Dental Research Center, Golestan University of Medical Sciences, Gorgan, Iran.
2Assistant Professor, Dental Research Center, Golestan University of Medical Sciences, Gorgan, Iran.
*
Correspondence to Elham Fakhari, Assistant professor, Dental Research Center, Golestan University of Medical Sciences, Gorgan, Iran. Tel: +989305818491, Email:
Fakhari_85@yahoo.com
Abstract
Background: The combination of chlorhexidine (CHX) and fluoride is believed to enhance the effects of both constituent elements, and reduce their possible side effects. This study aimed to evaluate the effect of CHX containing sodium fluoride on dental plaque, gingival inflammation, and tooth discoloration.
Methods: In this double-blind clinical study, 40 patients were selected and randomly divided into two groups. One group was given CHX 0.12%, and the other one was provided with sodium fluoride 0.05%-CHX 0.12% mouthwashes. Plaque index (PI), gingival index (GI), and discoloration index (DI) were measured at the beginning of the study and then after two weeks. Data were analyzed using chi-squared and independent t test.
Results: PI and GI were significantly reduced in the group with CHX + sodium fluoride compared to the one with CHX (P<0.001); however, the difference between two groups in terms of DI was not statistically significant (P =0.08). Both groups showed complications, but their differences were not statistically significant (P=0.5).
Conclusions: Mouth wash containing CHX + sodium fluoride was more effective in dental plaque control and gingival inflammation than the one only including CHX, although complications were not statistically significant between the two groups.
Keywords: Chlorhexidine, Dental plaque, Gingivitis, Sodium fluoride, Tooth discoloration
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Citation: Taghizadeh F, Fakhari E. Effect of Chlorhexidine Containing Fluoride on Dental Plaque, Gingivitis, and Tooth Discoloration: A Randomized Clinical Trial. Avicenna J Dent Res. 2020;13(2):67-71. doi: 10.34172/ajdr.2021.13.
Background
Highlights
Dental plaque is the major etiology of gingival inflammation (1). Although mechanical tooth cleaning is the most popular method for plaque removal, the use of chemical agents could prove useful to reduce deficiencies of toothbrushing methods, and facilitate control of gingivitis (2). Mouthrinses are the most frequently used chemical plaque control agents at home, and are available as means of delivering antiplaque ingredients in the oral cavity (3).
Chlorhexidine (CHX) has been established as the gold standard in chemical plaque control, but it produces some side effects such as staining of teeth and alteration in taste sensation (4). It is a biguanide agent, which damages the cellular transport of the bacterial cell in the lower concentration (1). It has also antimicrobial effects against periodontal and cariogenic pathogens (5).
Fluoride is an effective anti-caries agent mainly used in many oral health products, such as mouthwashes. It accumulates in dental plaque and reduces the amount of it and the gingival inflammation (5). Fluoride can be added to dental health products in various formulations. It has been indicated that the incorporation of sodium fluoride does not affect the availability of CHX in mouthwash formulations (4).
Since both CHX and fluoride have antibacterial activities and are effective agents against dental caries and gingivitis, it has been argued that their combination could have a synergistic effect (6). In the presence of fluoride, a lower concentration of CHX is needed, and their combination may have long-term effects compared to either of these mouthwashes applied separatly (4,7,8). In a systematic review, it has been shown that sodium fluoride could be added to CHX without reducing its anti-gingivitis effect.
Several dental health products containing the combination of fluoride and CHX, nowadays, are available in the markets (3). Taking into consideration the benefits of the two materials as well as the absence of related studies on Iranian CHX-containing mouthwashes, this study aimed to assess the effect of 0.12% CHX including sodium fluoride on dental plaque, gingivitis, and tooth discoloration.
Material and Methods
The present study was a randomized controlled clinical trial with a parallel, double-blind design. To carry out the study, 40 patients (17 men and 23 women) aged 18-50 referring to the School of Dentistry, Golestan University of Medical Sciences were included in it. The sample size was calculated according to the relevant study with a power of 90% and a significance level of 0.05 (9). Patients with at least 20 teeth (except third molars), who had a gingival index (GI) of ≤0.5 were included. The following subjects, on the other hand, were excluded from our study: (a) any patients with systemic disease that had negatively affected oral health, (b) those with history of periodontitis (the subjects had no site with probing pocket depth (PPD)>3 mm and had no site with clinical attachment loss (CAL)>2 mm), (c) those with pathological conditions in the mouth, (d) pregnant women or those breastfeeding, (e) those with untreated caries, (f) those with partial dentures or orthodontic appliances, (g) smokers, (h) those using CHX two weeks before or during the recruitment, (i) those receiving treatment with drugs that had influenced salivary flow or periodontal health within the earlier three months, and (j) those with a history of sensitivity to CHX.
The participants received professional tooth cleaning and oral hygiene instructions two weeks before using the study products; then, they were asked to perform the high standard plaque control procedures at home. All participants used the same toothpaste (Signal, Hamburg, Germany). At baseline (visit 1), the inclusion and exclusion criteria were assessed again and all study parameters were recorded. The participants were randomly divided into two groups: group A received 0.12% CHX (Donyaye Behdasht, Iran), and group B received 0.12% CHX+0.05% sodium fluoride (Emad Pharma, Iran). They were age- and sex-matched.
Another clinician who was not involved in recording the clinical parameters explained how to use the products. The participants were requested to use 10 mL of the mouthwashes for 1 min each morning and evening. A CONSORT-type diagram explaining the design of this study is presented in Figure 1.
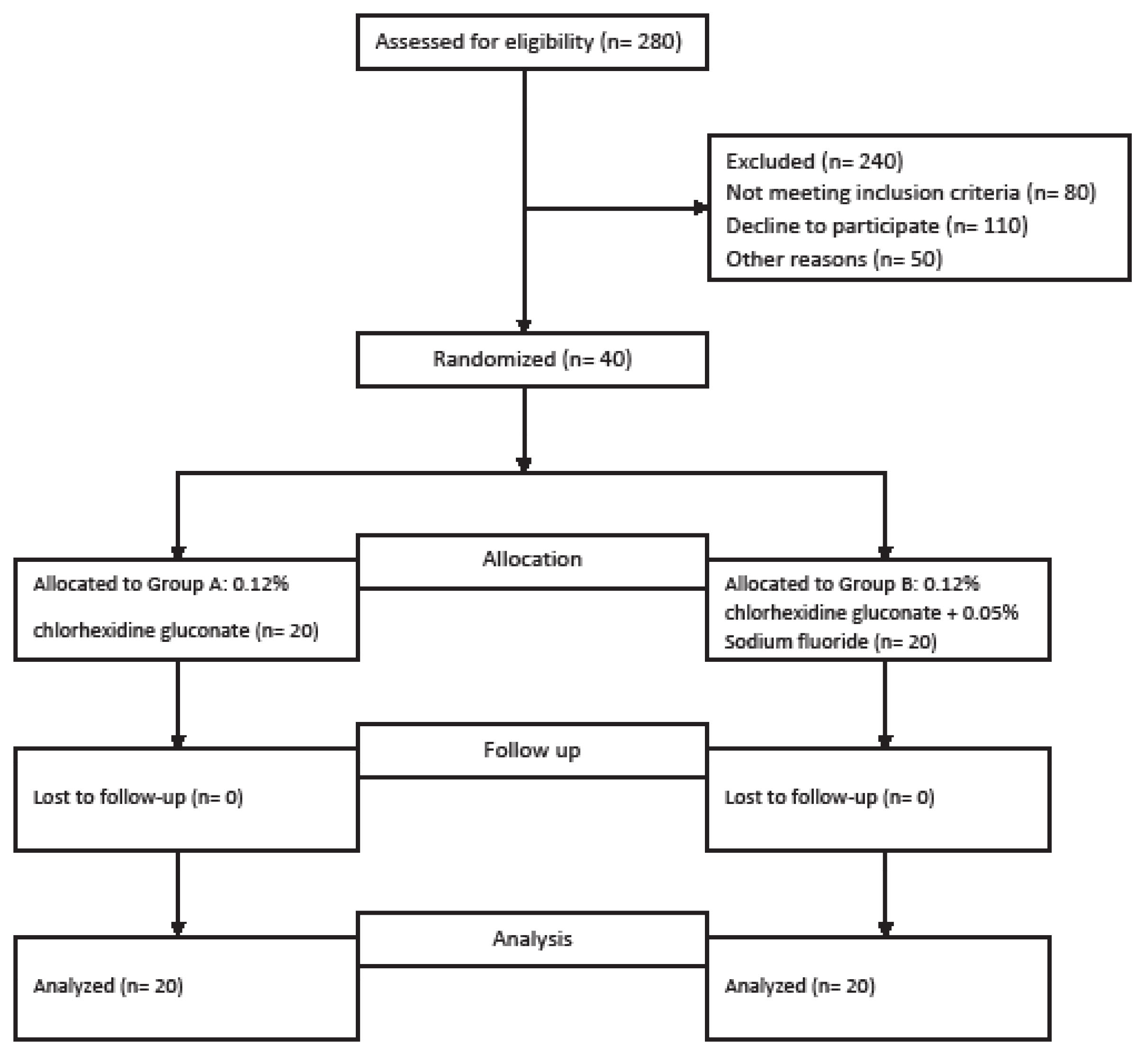
Figure 1.
CONSORT Diagram Depicting the Study Design.
.
CONSORT Diagram Depicting the Study Design.
The following parameters were assessed by a calibrated examiner (final year student) who was blinded to the group allocation of the patients at baseline and later 15 days. GI (10), plaque index (PI) (11), and discoloration index (DI) (12) were assessed. To investigate the examiner repeatability, 15 participants were selected and evaluated at baseline and after the study. The correlation coefficient for the indices was 0.89.
At the end of the study, all participants were asked to report their experiences. They were provided with a questionnaire in order to assess the impact of the mouthwash on their taste sensation. The questions were scored according to a scale from “−5” (extremely bad) to “+5” (extremely good). Moreover, they were requested to report the possible side effects of the study products. Their reported side effects were recorded as follows: short-term anesthesia, long-term anesthesia, blister, mild nausea, and none.
Statistical analysis was performed using SPSS software, version 16. Descriptive measures were calculated and reported as means and standard deviations. In our study, the independent t-test was applied to examine the statistical differences between clinical parameters in the two groups. Chi-square test was used to compare the proportion of participants reporting various adverse effects in the treatment groups. The significance level was set at 5%.
Results
According to our study results, 17 (42.5%) participants out of the 40 ones were men, and 23 (57.5%) were women, with a mean ± SD age of 30.60±6.70 years.
Statistically significant differences were found between the intervention groups regarding GI and PI at baseline and after 15 days (P < 0.001). It was detected that fluoride-containing CHX mouthwash significantly reduced gingival and plaque indices compared to those only containing CHX. There was no statistical difference between the two visits in terms of the discoloration index (P = 0.08, Table 1).
Table 1.
Descriptive Statistics for Indices at Baseline and After 14 Days in Each Group
|
Index
|
Baseline
Mean ± SD
|
After Intervention
Mean ± SD
|
Difference
Mean ± SD
|
P
Value
|
| Gingivitis |
|
|
|
<0.001 |
| Group A |
1.54±0.28 |
1.43±0.27 |
0.11±0.12 |
| Group B |
0.06±0.17 |
0.95±0.13 |
0.59±0.13 |
| Plaque |
|
|
|
<0.001 |
| Group A |
1.61±0.37 |
1.49±0.38 |
0.11±0.22 |
| Group B |
1.72±0.36 |
0.98±0.28 |
0.73±0.17 |
| Discoloration |
|
|
|
0.08 |
| Group A |
1±1.04 |
0.97±0.84 |
0.03±1.18 |
| Group B |
1.6±1.04 |
0.94±0.75 |
0.68±1.15 |
*SD: Standard deviation
Group A: 0.12% chlorhexidine; Group B: 0.12% chlorhexidine +0.05% sodium fluoride.
Statistical analysis showed no significant difference between the two types of mouthwash regarding taste sensation (P= 0.66, Table 2). There was no statistically significant difference between the two groups in terms of possible side effects (P = 0.53, Table 3).
Table 2.
Frequency (%) of the Effect on Taste Sensation After Mouthwash Use
|
Taste Sensation
|
Group A
|
Group B
|
| Very bad |
2 (10%) |
2 (10%) |
| Bad |
8 (40%) |
4 (20%) |
| Average |
7 (35%) |
8 (40%) |
| Good |
2 (10%) |
4 (20%) |
| Very good |
1 (5%) |
2 (10%) |
| Total |
20 (100%) |
20 (100%) |
Group A: 0.12% chlorhexidine; Group B: 0.12% chlorhexidine +0.05% sodium fluoride.
Table 3.
Frequency (%) of Side Effects After Mouthwashes Use
|
Side Effect
|
Group A
|
Group B
|
| Short-term anesthesia |
10 (50%) |
11 (55%) |
| Long-term anesthesia |
10 (50%) |
8 (40%) |
| Nausea |
0 (0%) |
1 (5%) |
| Total |
20 (100%) |
20 (100%) |
Group A: 0.12% chlorhexidine; Group B: 0.12% chlorhexidine +0.05% sodium fluoride.
Discussion
The daily use of antibacterial mouth rinse as a supplement for maintaining oral hygiene plays an important role in controlling dental plaque. CHX is a cationic antiseptic agent regarded as the ‘gold standard’ antiplaque substance, and is particularly effective against oral biofilm; however, its side effects limit its duration of use. CHX-containing fluoride mouth rinses have recently come onto the market as they inhibit dental caries and plaque, and promise to produce better hygienic results. The combination of these two mouth rinses into one single substance can no doubt facilitate their use (3).
Since the presence of CHX in a mouthwash does not guarantee a beneficial clinical effect of this formulation, the data about the clinical effect of different CHX products are comparable. In this study, the antiplaque and anti-gingivitis effects of two different formulations of CHX available in Iran’s market were compared. Moreover, extrinsic dental stains and possible reports of adverse effects were recorded.
In the present study, it was found that 0.12% CHX containing fluoride mouthwash significantly reduced bacterial plaque and gingivitis compared to CHX with no fluoride mouthwash. This finding was in line with a seven-day parallel study design with 0.2% CHX concentration. It should be noted that some studies had already failed to detect any difference between the two types of mouthwash (5,7). This discrepancy may be attributed to the differences in study design, target population, duration of the study, and various concentrations of CHX applied in the studies. A study by Barkvoll et al (13) found reduced availability of soluble CHX in the CHX+fluoride solution. The difference in the results might be explained by the in vitro conditions and the use of sodium monofluorophosphate, which may display different chemical properties comparing to sodium fluoride (6).
According to our study results, the side effects of fluoride-CHX mouthwash in the two types of mouthwash were not statistically different. No difference was found between CHX+sodium fluoride and CHX in terms of tooth discoloration, which was consistent with the results from a systematic review (8). Claydon et al (14) argued that CHX discoloration could act as a surrogate parameter of efficacy. They hypothesized that if discoloration was diminished, CHX could be inhibited. The absence of a significant difference regarding dental staining in previous studies (8) suggested that adding sodium fluoride had not negatively affected CHX activity; although one study finally found mouth rinses containing sodium fluoride to have had lower tooth discoloration (2).
The experimental gingivitis model (15) has been acknowledged as the best design to assess the antiplaque and anti-gingivitis effects of active components in mouth rinse, as shown in many clinical trials. The experimental phase in the present study lasted for 15 days. Löe et al stated that 10–21 days proved enough for the development of gingivitis in the lack of any mechanical plaque control methods (15). Before starting the experimental phase in our study, therefore, 14 days were assigned to guarantee the minimal presence of plaque and gingival inflammation.
Addy (16) showed that 0.2% CHX was capable of inhibiting the development of gingivitis when daily oral hygiene procedures were absent. In this study, 0.12% CHX concentration was used. There was no evidence that one concentration of CHX rinse was more effective than another. Although the plaque inhibition of CHX is dose-dependent and low-concentration rinse showing less effectiveness, some studies have demonstrated the efficacy of 0.06% CHX in reducing plaques (4).
The participants taking part in previous studies (17-19) were not representative of regular dental practice patients. Some of them were medical students, dental hygiene students, or orthodontic patients. The participants included in this study, on the other hand, were selected from among regular dental patients with a mean ± SD age of 30.60±6.70 years.
Our study results also demonstrated thata combination of anti-caries and antiplaque agents may have been useful, yielded more benefits, and proved valuable in the prevention of periodontal disease. The combinations of fluoride and CHX mouthwashes made in Iran was applied in our study so that it could be recommended where required.
The limitations of this study included its small sample size and short follow-up period. Another limitation of our study was its failure to assess the microbiological effect of the mouthwashes. Therefore, it is recommended that further studies be carried out to address the given limitations.
Conclusions
It was concluded that combining CHX and sodium fluoride would improve the antiplaque and anti-gingivitis effect of CHX. Furthermore, no variation was observed in the development of tooth discoloration.
Conflict of Interest Disclosures
The authors declare that they have no competing financial interests or personal relationships that could influence the work reported in this study.
Acknowledgments
The authors gratefully acknowledge the financial support of Golestan University of Medical Sciences.
Ethical Statement
Ethical approval was obtained from the Ethics Committee of Golestan University of Medical Sciences (IR.goums.REC.1395.145), and all the participants signed an informed consent form. The clinical trial registry number was IRCT2016110630744N1.
Authors’ Contribution
EF conceived the ideas and methodology, collected the data, interpreted the data, led the writing and editing, and obtained the final approval of the manuscript. FT collected, analyzed, and interpreted the data, and obtained the final approval of the manuscript.
Funding
This research was financially supported by Golestan University of Medical Sciences.
References
- Franco Neto CA, Parolo CC, Rösing CK, Maltz M. Comparative analysis of the effect of two chlorhexidine mouthrinses on plaque accumulation and gingival bleeding. Braz Oral Res 2008; 22(2):139-44. doi: 10.1590/s1806-83242008000200008 [Crossref] [ Google Scholar]
- Kumar S, Patel S, Tadakamadla J, Tibdewal H, Duraiswamy P, Kulkarni S. Effectiveness of a mouthrinse containing active ingredients in addition to chlorhexidine and triclosan compared with chlorhexidine and triclosan rinses on plaque, gingivitis, supragingival calculus and extrinsic staining. Int J Dent Hyg 2013; 11(1):35-40. doi: 10.1111/j.1601-5037.2012.00560.x [Crossref] [ Google Scholar]
- Shukla N, Saha S, Singh S. Effect of chlorhexidine with fluoride mouthrinse on plaque accumulation, plaque pH-a double blind parallel randomized clinical trial. J Clin Diagn Res 2016; 10(7):ZC62-5. doi: 10.7860/jcdr/2016/18080.8186 [Crossref] [ Google Scholar]
- Sadat Sajadi F, Moradi M, Pardakhty A, Yazdizadeh R, Madani F. Effect of fluoride, chlorhexidine and fluoride-chlorhexidine mouthwashes on salivary Streptococcus mutans count and the prevalence of oral side effects. J Dent Res Dent Clin Dent Prospects 2015; 9(1):49-52. doi: 10.15171/joddd.2015.010 [Crossref] [ Google Scholar]
- Dehghani M, Abtahi M, Sadeghian H, Shafaee H, Tanbakuchi B. Combined chlorhexidine-sodiumfluoride mouthrinse for orthodontic patients: clinical and microbiological study. J Clin Exp Dent 2015; 7(5):e569-75. doi: 10.4317/jced.51979 [Crossref] [ Google Scholar]
- Villa O, Ramberg P, Fukui H, Emilson CG, Papanikolaou G, Heijl L. Interaction between chlorhexidine and fluoride in a mouthrinse solution-a 4-day and 6-week randomized clinical pilot study. Clin Oral Investig 2018; 22(3):1439-48. doi: 10.1007/s00784-017-2219-7 [Crossref] [ Google Scholar]
- Lorenz K, Bruhn G, Heumann C, Netuschil L, Brecx M, Hoffmann T. Effect of two new chlorhexidine mouthrinses on the development of dental plaque, gingivitis, and discolouration A randomized, investigator-blind, placebo-controlled, 3-week experimental gingivitis study. J Clin Periodontol 2006; 33(8):561-7. doi: 10.1111/j.1600-051X.2006.00946.x [Crossref] [ Google Scholar]
- Elkerbout TA, Slot DE, Van Loveren C, Van der Weijden GA. Will a chlorhexidine-fluoride mouthwash reduce plaque and gingivitis?. Int J Dent Hyg 2019; 17(1):3-15. doi: 10.1111/idh.12329 [Crossref] [ Google Scholar]
- Solís C, Santos A, Nart J, Violant D. 02% chlorhexidine mouthwash with an antidiscoloration system versus 02% chlorhexidine mouthwash: a prospective clinical comparative study. J Periodontol 2011; 82(1):80-5. doi: 10.1902/jop.2010.100289 [Crossref] [ Google Scholar]
- Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol 1967; 38(6):610-6. doi: 10.1902/jop.1967.38.6.610 [Crossref] [ Google Scholar]
- Silness J, Löe H. Periodontal disease in pregnancy II Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 1964; 22:121-35. doi: 10.3109/00016356408993968 [Crossref] [ Google Scholar]
- Brecx M, Macdonald LL, Legary K, Cheang M, Forgay MG. Long-term effects of Meridol and chlorhexidine mouthrinses on plaque, gingivitis, staining, and bacterial vitality. J Dent Res 1993; 72(8):1194-7. doi: 10.1177/00220345930720080601 [Crossref] [ Google Scholar]
- Barkvoll P, Rölla G, Bellagamba S. Interaction between chlorhexidine digluconate and sodium monofluorophosphate in vitro. Scand J Dent Res 1988; 96(1):30-3. doi: 10.1111/j.1600-0722.1988.tb01404.x [Crossref] [ Google Scholar]
- Claydon NC, Addy M, Adams G, Smith SR, Bosma ML, North M. A comparison of two chlorhexidine gel brushing regimens and a conventional toothpaste brushing regimen for the development of tooth staining over a 6-week period. Int J Dent Hyg 2006; 4(4):183-8. doi: 10.1111/j.1601-5037.2006.00211.x [Crossref] [ Google Scholar]
- Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol 1965; 36:177-87. doi: 10.1902/jop.1965.36.3.177 [Crossref] [ Google Scholar]
- Addy M. Chlorhexidine compared with other locally delivered antimicrobials A short review. J Clin Periodontol 1986; 13(10):957-64. doi: 10.1111/j.1600-051x.1986.tb01434.x [Crossref] [ Google Scholar]
- Mendieta C, Vallcorba N, Binney A, Addy M. Comparison of 2 chlorhexidine mouthwashes on plaque regrowth in vivo and dietary staining in vitro. J Clin Periodontol 1994; 21(4):296-300. doi: 10.1111/j.1600-051x.1994.tb00321.x [Crossref] [ Google Scholar]
- Quirynen M, Avontroodt P, Peeters W, Pauwels M, Coucke W, van Steenberghe D. Effect of different chlorhexidine formulations in mouthrinses on de novo plaque formation. J Clin Periodontol 2001; 28(12):1127-36. doi: 10.1034/j.1600-051x.2001.281207.x [Crossref] [ Google Scholar]
- Hoffmann T, Bruhn G, Richter S, Netuschil L, Brecx M. Clinical controlled study on plaque and gingivitis reduction under long-term use of low-dose chlorhexidine solutions in a population exhibiting good oral hygiene. Clin Oral Investig 2001; 5(2):89-95. doi: 10.1007/s007840100114 [Crossref] [ Google Scholar]