Avicenna J Dent Res. 15(3):134-137.
doi: 10.34172/ajdr.1612
Case Report
The Second Case Report of Cellular Cannibalism in Ameloblastoma
Massoumeh Zargaran 1, *  , Shokoofeh Jamshidi 2
, Shokoofeh Jamshidi 2  , Mohiadin Amjadian 3
, Mohiadin Amjadian 3 
Author information:
1Associate Professor, Department of Oral and Maxillofacial Pathology, Faculty of Dentistry, Kurdistan University of Medical Sciences, Sanandaj, Iran
2Associated Professor, Dental Research Center, Department of Oral and Maxillofacial Pathology, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran
3Assistant Professor, Department of English Language, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran
Abstract
Cellular cannibalism (CC) is a phenomenon during which the tumor cell engulfs other cells, including sibling tumor cells or non-tumor cells such as neutrophils and lymphocytes (known as Xeno-CC). This case report aimed to present CC in three ameloblastomas. Microscopically, hematoxylin and eosin-stained slides of 14 ameloblastoma cases were observed at 400× magnification to identify CC. The findings revealed that 3 cases (21.42 %), including 2 plexiform and 1 follicular ameloblastoma were identified with CC and neutrophil Xeno-CC in stellate reticulum-like cells. Furthermore, a few CC were seen in peripheral ameloblast-like tumor cells. CC has been described as an exclusive morphologic feature of malignancies; however, since it has been reported in benign tumor cells such as ameloblastoma, it seems that this concept needs to be revised. Given the relation between CC and aggressiveness of tumor lesions, this phenomenon can be suggested as one of the predicting factors for aggressive biologic behavior of ameloblastoma.
Keywords: Ameloblastoma, Cannibalism, Cannibalistic cell, Odontogenic tumor
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Zargaran M, Jamshidi S, Amjadian M. The second case report of cellular cannibalism in ameloblastoma. Avicenna J Dent Res. 2023; 15(3):134-137. doi:10.34172/ajdr.1612
Background
Cannibalism (derived from the Spanish word “Cannibal”) means eating another human flesh by human as food (1). Similarly, this phenomenon is seen at the cellular level (2) when an alive tumor cell is engulfed (3) and digested by another tumor cell (4) and also dies inside it (3). In cellular cannibalism (CC), first, a cannibalistic cell engulfs a free cell (5). The engulfed cell contains a large vacuole that can push the cannibalistic cell’s nucleus to the cell borders and make a transformation in its shape from circular to semi-lunar (4,5). Engulfed cell’s nucleus shape does not change (5). This cell lives for a while (1), it but gradually turns into an apoptotic cell (6), dies, and its nucleus disintegrates (1). CC was mainly known as a phenomenon in which the tumor cell engulfs its sibling tumor cell. However, engulfing other types of cells such as neutrophils and lymphocytes has recently been reported (7,8,9) which is called Xeno-CC (9). Generally, CC has been described as a characteristic morphological feature of malignancy (5,9,10), but lately, it has been reported to occur in benign pathologic lesions (3,6,11-13). The aim of the present study was to report CC in three ameloblastoma cases.
Case Presentation
Cases
We conducted a retrospective study on 14 ameloblastoma cases (retrieved from the department of oral pathology, Faculty of Dentistry, Hamadan University of Medical Sciences) to identify CC. The classical feature of CC was defined by the eccentrically placed crescent-shaped nucleus and cytoplasmic extensions, which partially (partial CC) or completely (complete CC) surround the internalized cell with a clear halo representing a vacuole formation (9,12). Based on the previous studies (6-8,14) and according to the sequential phases in the course of CC in which the internalized cell dies off, and its nucleus eventually undergoes degeneration (1,5), other histopathologic features of this phenomenon (e.g., vacuole including the degrading cell, empty vacuole, and complex cannibalism) have been considered as CC if present. Then, the presence of Xeno-CC was checked, and demographic data of the samples were collected from patients’ records (Table 1).
Table 1.
Clinical-Radiographical and Histopathological Data of Studied Ameloblastoma Cases
|
Case No.
|
Age
|
Gender
|
Jaw Involved
|
Clinical Feature (Sign/Symptom) |
Radiographical Feature
|
Type of Histopathology
|
CC
|
| 1 |
22 |
Male |
Mandible |
- |
Multicystic |
Plexiform |
+
|
| 2 |
34 |
Male |
Mandible |
Expansion |
Multicystic |
Plexiform |
+
|
| 3 |
25 |
Female |
Mandible |
Perforation |
Multicystic |
Follicular |
+
|
| 4 |
18 |
Female |
Mandible |
Expansion |
Unicystic |
- |
-
|
| 5 |
22 |
Male |
Mandible |
Expansion |
Multicystic |
Plexiform |
-
|
| 6 |
21 |
Female |
Mandible |
Expansion |
Unicystic |
- |
-
|
| 7 |
24 |
Male |
Mandible |
Expansion |
Unicystic |
- |
-
|
| 8 |
25 |
Male |
Mandible |
Tooth mobility |
Multicystic |
Follicular |
-
|
| 9 |
33 |
Female |
Mandible |
- |
Unicystic |
- |
-
|
| 10 |
26 |
Female |
Mandible |
Expansion |
Multicystic |
Plexiform |
-
|
| 11 |
47 |
Male |
Maxilla |
- |
Multicystic |
Plexiform |
-
|
| 12 |
38 |
Female |
Mandible |
Pain |
Unicystic |
- |
-
|
| 13 |
34 |
Male |
Mandible |
Expansion |
Multicystic |
Follicular |
-
|
| 14 |
53 |
Female |
Mandible |
Expansion, Pain |
Unicystic |
- |
-
|
Note. CC: Cellular cannibalism.
Histopathologic Findings
In microscopic assessment, both CC and Xeno-CC were detected in 3 (21.42%) cases, including 2 cases of plexiform and 1 follicular ameloblastoma. In these samples, CC and neutrophil Xeno-CC were clearly observed in the stellate reticulum (SR)-like cells (Figures 1 and 2), and a few CC were found in peripheral ameloblastic-like cells (Figure 3).
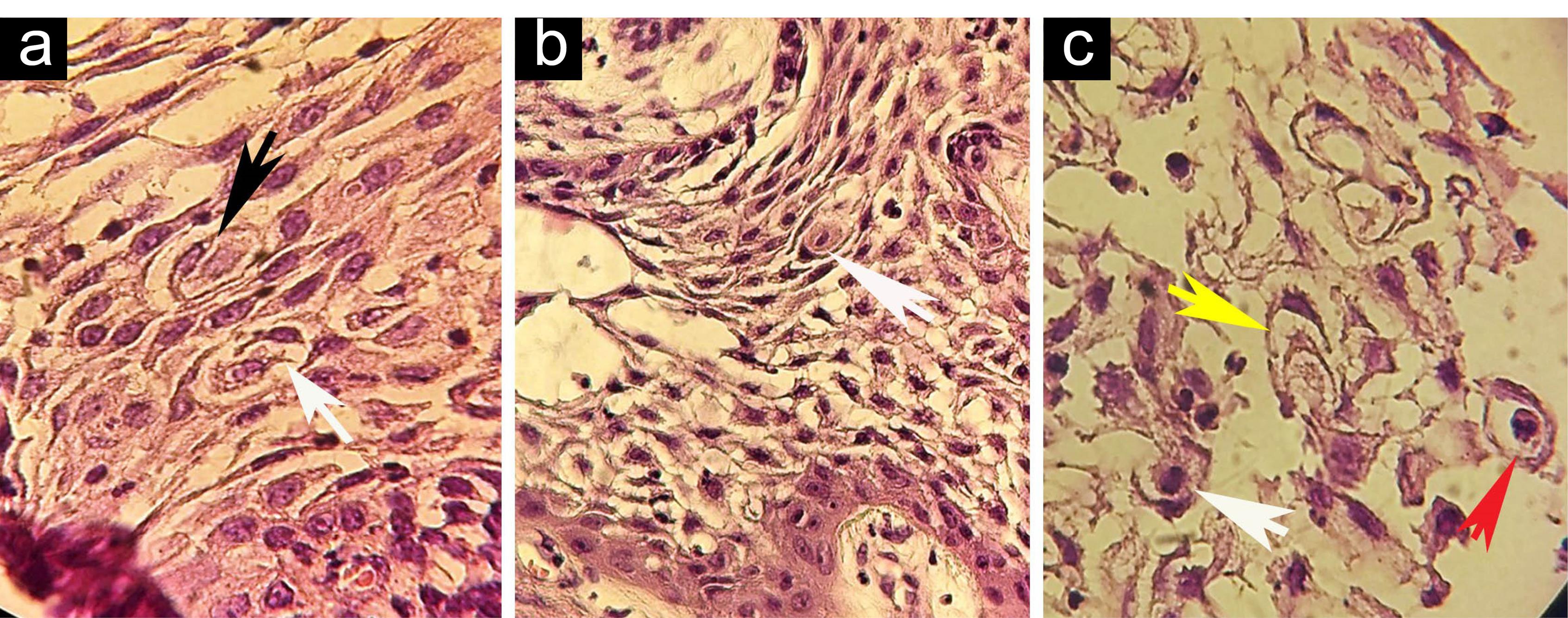
Figure 1.
(a-c) Complete CC (black arrowhead), Partial CC (white arrowheads), Complex CC (yellow arrowhead), and neutrophil Xeno- CC (red arrowhead); H&E, ×400. Note. CC: Cellular cannibalism.
.
(a-c) Complete CC (black arrowhead), Partial CC (white arrowheads), Complex CC (yellow arrowhead), and neutrophil Xeno- CC (red arrowhead); H&E, ×400. Note. CC: Cellular cannibalism.
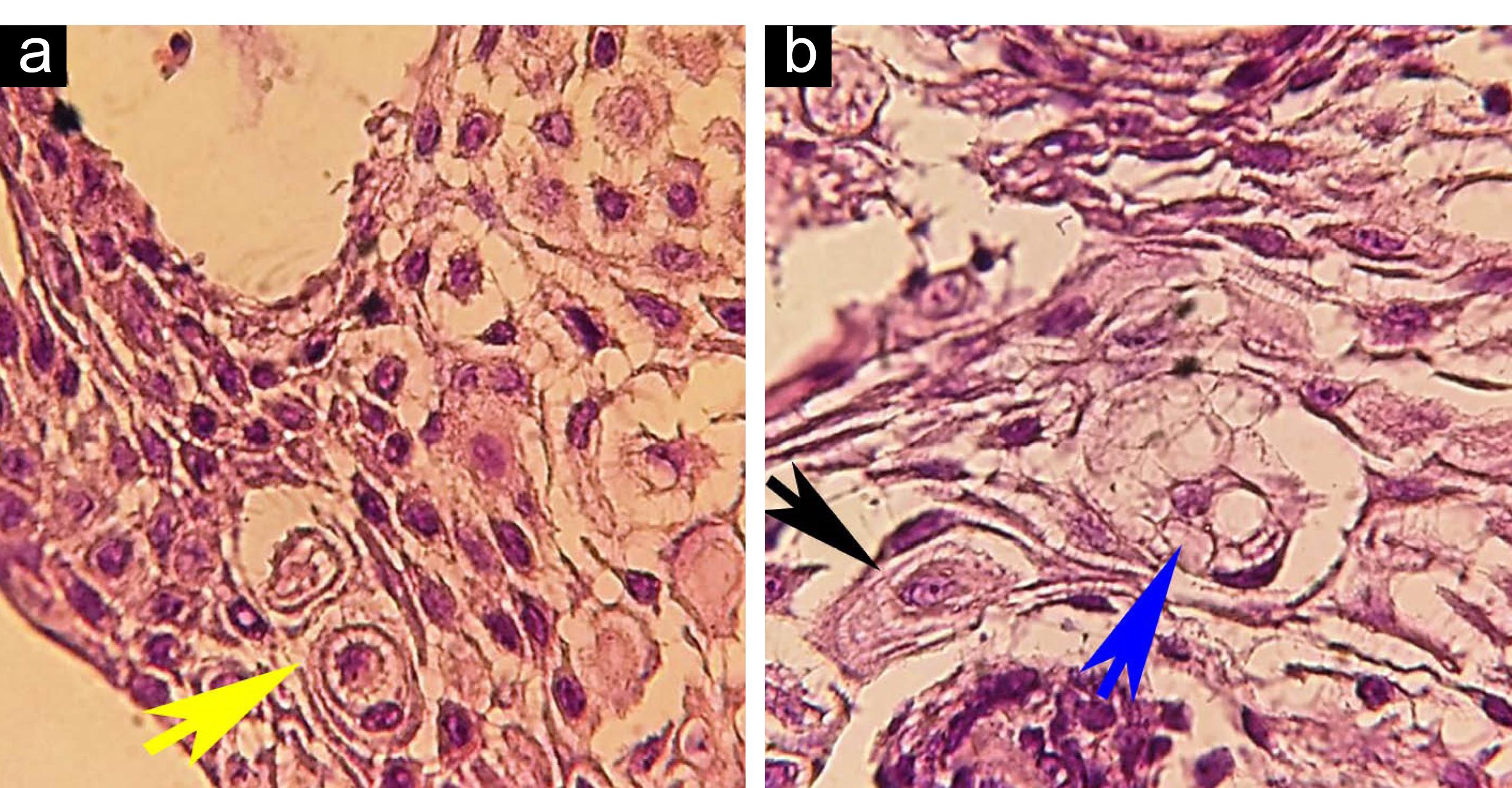
Figure 2.
(a) CC. (b) CC With multiple vacuoles cannibalism (blue arrowhead), complete CC (black arrowhead); H&E, ×400. Note. CC: Cellular cannibalism.
.
(a) CC. (b) CC With multiple vacuoles cannibalism (blue arrowhead), complete CC (black arrowhead); H&E, ×400. Note. CC: Cellular cannibalism.
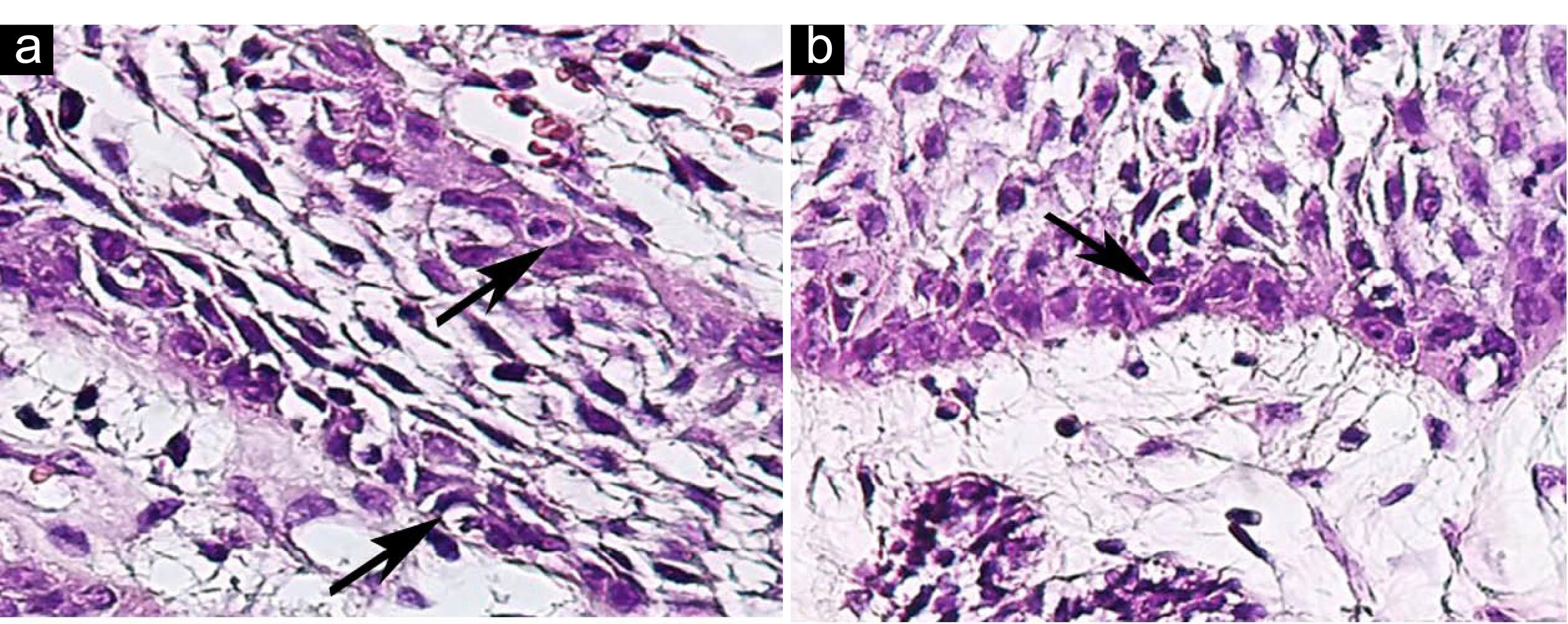
Figure 3.
(a-b) CC in Peripheral Ameloblast-like Cells of Ameloblastoma; H&E, ×400. Note. CC: Cellular cannibalism.
.
(a-b) CC in Peripheral Ameloblast-like Cells of Ameloblastoma; H&E, ×400. Note. CC: Cellular cannibalism.
Discussion
With the identification of CC in malignant tumor cells (5,9), its relationship with aggressive behavior (5,8,10), grade of anaplasia (15), and metastatic potential of malignancy (10) was reported as well. It has been mentioned that in this phenomenon, internalized cells disrupt the cytokinesis of engulfing cells, consequently causing a chromosomal instability and inducing aneuploidy which promotes tumor progression (8). Therefore, CC was suggested as a prognostic marker for predicting biological behavior and aggressiveness of tumor lesions (5,10), and it was described as a characteristic feature for malignant tumors compared to benign tumors (9). Recently, CC has been reported in benign lesions such as ameloblastoma (12), giant cell tumor of the tendon sheath, central giant cell granuloma (GCG), peripheral GCG. Further, its relation with aggressive biologic behavior of some of these lesions has been clarified (3,11-13). For example, more CC has been reported in giant cells of tendon sheath than in those of central GCG and peripheral GCG (6). In addition, the number of reported CC in giant cells of the aggressive and recurrent central GCGs was higher than in non-aggressive and non-recurrent central GCGs (6,11,13). However, a hypothesis declared that the cannibalistic activity of giant cells in these lesions cannot be considered a true CC as these cells are derived from monocyte-macrophage lineage, and their engulfing activity is due to their inherent phagocytic nature (12). Hence, it seems that the first report of true CC in a benign human tumor is CC in SR-like cells of ameloblastoma which was first presented by Sarode et al (12), and the present study is its second report. This study is the first report of CC in peripheral ameloblast-like cells and neutrophil-Xeno CC in SR-like cells of ameloblastoma. In this study, CC was observed in 3 cases (21.42%) of ameloblastoma which was lower than that in Sarode and colleagues’ study (30%) (12). All 3 positive CC ameloblastomas were conventional ameloblastomas with plexiform (2 cases) and follicular (1 case) histopathologic patterns, but in Sarode et al’ s report (12), positive CC cases were follicular (n=10), acanthomatous (n=2), and unicystic (n=3) ameloblastomas. As no clinical and radiographic findings were reported by Sarode et al (12), we could not compare them with our findings. Similar to Sarode et al’s study (12), the current study found partial and complete CC features, along with other features such as vacuoles including degrading cell(s), CC with multiple/empty vacuoles, complex cannibalism, and neutrophil Xeno-CC. The definite reason for CC occurrence is not clear (4,10); however, several theories have been raised: First, through this phenomenon, tumor cells can feed themselves from engulfed cells and meet their hunger as well as nutritional deficiencies (1,10). Secondly, CC prevents tumor growth by elimination and reduction of tumor cells (4,10). Thirdly, the occurrence of CC can be due to an unfavorable microenvironment such as acidic conditions in the lesion. The acidic microenvironment undergoes a shift in the metabolic pathway which in turn favors the selection of certain cell phenotypes that can act as cannibalistic cells (10). Finally, tumor cells use CC as an escape mechanism for escaping from immunity through immune cell cannibalism (1,10).
As a result of ameloblastoma growth progression and the increase in the size of its components, central SR-like cells of follicles (and plexiforms) get away from stroma, which is counted as a nutritional and blood supply source for the cells, so CC can be a surviving strategy in a microenvironment without nutrients. Stroma degeneration is often mentioned as a marker of nutritional deficiency (12). In the present study, the detection of CC in peripheral ameloblast-like cells neighboring the stroma may be an indicator of degeneration in this nutritional source, indirectly. In addition, CC has been considered a sign of aggressive behavior. In this regard, Sarode et al suggested that CC can approve the aggressive nature of ameloblastoma and be used for predicting its biological behavior and prognosis (12); however, two points need to be considered: In the study by Sarode et al (12), clinical and radiographic features of ameloblastomas were not presented, and their relation with CC was not investigated. Conversely, in the present study, the frequency of clinical and radiographic features was higher in ameloblastomas without CC (90.90%, 10 out of 11 cases) compared with positive CC ameloblastomas (66%, 2 out of 3 cases).
Conclusions
Regarding the relation between CC and the aggressiveness of tumor lesions, the presence of CC in ameloblastoma may be suggested as one of the predictive factors for the aggressive biologic behavior of this tumor. A definite conclusion on this topic involves assessing the relationship between clinical and radiologic features and the presence of CC in these lesions. Accordingly, further studies with a higher sample size are required.
Authors’ Contribution
Conceptualization: Massoumeh Zargaran.
Data curation: Massoumeh Zargaran, Shokoofeh Jamshidi.
Formal analysis: Massoumeh Zargaran, Shokoofeh Jamshidi.
Investigation: Massoumeh Zargaran, Shokoofeh Jamshidi, Mohiadin Amjadian.
Methodology: Massoumeh Zargaran.
Project administration: Massoumeh Zargaran.
Supervision: Massoumeh Zargaran.
Validation: Massoumeh Zargaran, Shokoofeh Jamshidi.
Visualization: Massoumeh Zargaran, Shokoofeh Jamshidi, Mohiadin Amjadian.
Writing–original draft: Massoumeh Zargaran, Shokoofeh Jamshidi, Mohiadin Amjadian.
Writing–review & editing: Massoumeh Zargaran, Shokoofeh Jamshidi, Mohiadin Amjadian.
Competing Interests
The authors declare no conflict of interests.
Ethical Approval
This study was approved by the Research Ethics Committee of the Kurdistan University of Medical Sciences (IR.MUK.REC.1399.009).
References
- Sharma N, Dey P. Cell cannibalism and cancer. Diagn Cytopathol 2011; 39(3):229-33. doi: 10.1002/dc.21402 [Crossref] [ Google Scholar]
- Yang YQ, Li JC. Progress of research in cell-in-cell phenomena. Anat Rec (Hoboken) 2012; 295(3):372-7. doi: 10.1002/ar.21537 [Crossref] [ Google Scholar]
- Fernandez-Flores A. Cannibalism in a benign soft tissue tumor (giant-cell tumor of the tendon sheath, localized type): a study of 66 cases. Rom J Morphol Embryol 2012; 53(1):15-22. [ Google Scholar]
- Arya P, Khalbuss WE, Monaco SE, Pantanowitz L. Salivary duct carcinoma with striking neutrophil-tumor cell cannibalism. Cytojournal 2011; 8:15. doi: 10.4103/1742-6413.84222 [Crossref] [ Google Scholar]
- Abodief WT, Dey P, Al-Hattab O. Cell cannibalism in ductal carcinoma of breast. Cytopathology 2006; 17(5):304-5. doi: 10.1111/j.1365-2303.2006.00326.x [Crossref] [ Google Scholar]
- Sarode GS, Sarode SC, Gawande S, Patil S, Anand R, Patil SG. Cellular cannibalism in giant cells of central giant cell granuloma of jaw bones and giant cell tumors of long bones. J Investig Clin Dent 2017; 8(2):e12214. doi: 10.1111/jicd.12214 [Crossref] [ Google Scholar]
- Lugini L, Matarrese P, Tinari A, Lozupone F, Federici C, Iessi E. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res 2006; 66(7):3629-38. doi: 10.1158/0008-5472.can-05-3204 [Crossref] [ Google Scholar]
- Sarode SC, Sarode GS. Neutrophil-tumor cell cannibalism in oral squamous cell carcinoma. J Oral Pathol Med 2014; 43(6):454-8. doi: 10.1111/jop.12157 [Crossref] [ Google Scholar]
- Gupta K, Dey P. Cell cannibalism: diagnostic marker of malignancy. Diagn Cytopathol 2003; 28(2):86-7. doi: 10.1002/dc.10234 [Crossref] [ Google Scholar]
- Jose D, Mane DR, Datar U, Muttagi S, Hallikerimath S, Kale AD. Evaluation of cannibalistic cells: a novel entity in prediction of aggressive nature of oral squamous cell carcinoma. Acta Odontol Scand 2014; 72(6):418-23. doi: 10.3109/00016357.2013.798872 [Crossref] [ Google Scholar]
- Sarode SC, Sarode GS. Cellular cannibalism in central and peripheral giant cell granuloma of the oral cavity can predict biological behavior of the lesion. J Oral Pathol Med 2014; 43(6):459-63. doi: 10.1111/jop.12119 [Crossref] [ Google Scholar]
- Sarode GS, Sarode SC, Gadbail AR, Gondivkar S, Patil S. Histomorphological evidence of cellular cannibalism in ameloblastoma. J Contemp Dent Pract 2019; 20(7):863-6. [ Google Scholar]
- Urs AB, Yaming P, Malhotra R. An insight into the cannibalistic behavior of giant cell granulomas of the jaws. J Oral Maxillofac Pathol 2018; 22(3):449. doi: 10.4103/jomfp.JOMFP_67_18 [Crossref] [ Google Scholar]
- Sarode GS, Sarode SC, Karmarkar S. Complex cannibalism: an unusual finding in oral squamous cell carcinoma. Oral Oncol 2012; 48(2):e4-6. doi: 10.1016/j.oraloncology.2011.08.013 [Crossref] [ Google Scholar]
- Hattori M, Nishino Y, Kakinuma H, Matsumoto K, Ohbu M, Okayasu I. Cell cannibalism and nucleus-fragmented cells in voided urine: useful parameters for cytologic diagnosis of low-grade urothelial carcinoma. Acta Cytol 2007; 51(4):547-51. doi: 10.1159/000325792 [Crossref] [ Google Scholar]